DIY powder for dishwashers: we disassemble industrial products and improve the recipe

Attention! In the recipe with bleach, there was a danger of metal corrosion! Not recommended in standard use!
Experimental review a year later:
DIY powder for dishwashers: how not to dissolve the dishes and not repeat my mistakes. Year of experiments
In the last publication, we created a cheap powder for dishwashers from
Why am I doing this at all?
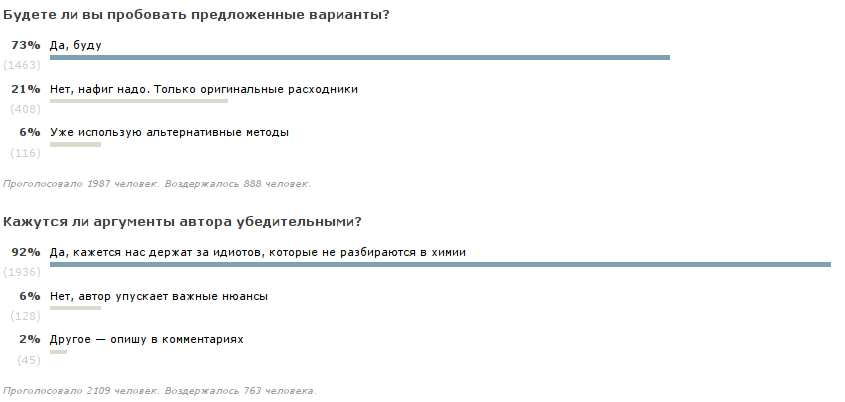
It's fun. This is a powerful argument for me. The second weighty argument for a more detailed study of the topic was the reaction of the audience. I did not expect so many people would be interested. Moreover, judging by the results of the survey, the majority will either try recipes or already use non-standard means. And I was surprised at how they pulled the publication on the RuNet. Either the crisis has affected, or the topic is relevant. And often these are not only near-tech communities like ixbt, but also numerous women's forums. Therefore, I want to ask everyone who has already experimented in this direction to share their results, recipes and places where you can get the components.
Industrial detergents
Deciding to modify the detergent, I turned to industrial recipes. Sound logic suggested that large food factories or hotels would not spend money on retail boxes and bottles. I was especially interested in the CIP-cleaning method (Cleaning in Place - CIP) - cleaning by heating and circulating washing solutions inside the processing equipment without the need for its analysis. Classic examples are washing containers at dairies, all kinds of ketchup / mayonnaise pipelines in food production. Having decided that the product that successfully peels dried mayonnaise out of the factory would suit us, I began to rummage through the compositions. Take, for example, our manufacturer of NPF Himitek from St. Petersburg. The range is simply huge for all possible appointments. Shipped as a rule
 Khimitek MIRACLE-CIP
Khimitek MIRACLE-CIP Concentrated liquid foamless alkaline degreaser for the food industry
Designed for alkaline CIP-washing of food processing equipment. Removes oil and protein contaminants . It is used to clean effluents from organic pollutants. It is recommended for use at food facilities, catering establishments and in other institutions of various profiles.
Areas of use:
- food and processing industry enterprises;
- cafes, bars, restaurants and other catering establishments;
- medical institutions;
- preschool and educational institutions;
- sanatorium establishments;
- real estate and utilities;
- shopping and entertainment centers and business centers;
- other enterprises and institutions of various profiles.
And now the drum roll! The incredible complexity of the components. Composition:
Alkali, complexing agents, water.
And here is a ready-made option for a dishwasher:
 HIMITEK KUKHMASTER-PROFI
HIMITEK KUKHMASTER-PROFI concentrated liquid low-foam alkaline detergent for dishwashers of any type
Designed for automatic washing dishes in soft and medium hard water. Removes oil, protein, starchy and other organic contaminants. It does not have a destructive effect on surfaces made of plastic and rubber. Prevents the formation of calcium plaque. It is recommended for use at public catering establishments, as well as at catering facilities of enterprises and institutions of any profile, including catering facilities of medical facilities for dishes that do not require special sanitary treatment, in sanatorium-resort, child care, preschool, school institutions, etc.
Composition:
Alkali 15–30%, sodium tripolyphosphate 5–15%, EDTA (complexing agent) 5–15%, inorganic salts 5–15%, polycarboxylate <5%, water.
Option for hard water:
 KHIMITEK KUHMASTER-PROFI 12 ° F
KHIMITEK KUHMASTER-PROFI 12 ° Fconcentrated liquid low-foam alkaline detergent for dishwashers of all types
Designed for automatic washing dishes in water of any degree of hardness up to 12 ° W (GOST R 52029-2003). Removes oil, protein, starchy and other organic contaminants. It does not have a destructive effect on surfaces made of plastic and rubber. Prevents the formation of calcium plaque. It is recommended for use at the enterprises of the food industry, public catering, as well as at the catering facilities of enterprises and institutions of various profiles.
Composition:
Alkali 5–15%, EDTA (complexing agent) 15–30%, inorganic salts <5%, water.
All of the above is directly intended for everything in a row, including catering and more. The composition is simple to disgrace. Alkali as the main component that decomposes fat and protein contaminants, and auxiliary components. It is already sufficient for washing that the pH is highly alkaline (pH> 11).
Also, various surfactant variants (surfactants) are often additionally used, which facilitate the separation of pollution and prevent its resorption back to the surface. To increase the effectiveness of surfactants in hard water, complexing agents that bind calcium and magnesium ions are used.
In fact, we have already decided on the minimum necessary kit. For washing dishes, it is still very desirable to add an oxidizing agent to discolor tea, coffee, juices and other things.
Chemical bleaches
Caution - risk of corrosion. Identified as a result of lengthy tests.
Bleaching agents are inherently oxidizing agents. In everyday life and industry, two main groups are used - chloric and oxygen .
Chloric are more aggressive and are used, as a rule, when guaranteed disinfection is required. Most often it is sodium hypochlorite with a surfactant. For example, toilet cleaners. Chlorine is a halogen, therefore it is rather aggressive in relation to metals. When used on a fabric, it significantly damages the fiber, leaves terrible spots due to discoloration of the colors on the fabric. Pluses directly follow from its shortcomings - eats almost any dyes and pigments, including pomegranate juice or blueberries. It’s not very suitable for us because of our attitude to metals. Single use of the machine will not hurt, but you can use softer means.
Oxygen bleaches are usually built on the basis of various peroxides, which are unstable compounds and easily give off oxygen in the presence of organic matter. For example, the well-known hydrogen peroxide - H2 O 2 . For our purposes, we will take sodium percarbonate (aka persol), which is a compound of sodium carbonate (washing soda) and hydrogen peroxide. Under normal conditions, this compound is fairly stable, but with the addition of TAED (TetraAcetylEthyleneDiamine), the percarbonate begins to work from 30 degrees.
In the dishwasher, this is very handy. At the first stage, everything hisses beautifully and foams. Oxygen mechanically tears off particles of dirt and at the same time oxidizes everything that can. At the second stage, sodium carbonate Na 2 CO 3 , remaining from the decomposition of percarbonate, turns the solution into hot alkaline hell with a pH of ≈ 11.5. We examined the properties of washing soda in a previous publication.
The main problem is where to buy? There are powder bleaches for washing machines, such as the same Eared Nanny. Cheap. But the composition contains a surfactant and optical brightener. We do not need surfactants, we will add them separately, but here they will only create excessive foam for us. We also do not need optical brightener. There is nothing fatal in it, but the microparticles of the fluorescent dye stuck on the dishes are clearly superfluous. And here a problem arises. A traditional feature of modern products - an unspoiled product is more expensive! That is, they didn’t make the components, called the result an eco-bio-vegan-gay friend formula, and increased the price by 10 times. The whole market is littered with similar crap from castrated formulas of powders, soap nuts and the like.

We will go the other way. There is a supermarket chain from the series all at 49 rubles FixPrice . Going there is a little uncomfortable, they are very specific, but there is a wonderful oxygen bleach under the Green Price brand , 49 rubles for 500 g.
Ingredients: Sodium carbonate peroxyhydrate (this is the same percarbonate)> 30%, sodium carbonate (soda ash)> 30%, TAED <5% (activator). I bought 4 packs and dragged away to test.
About hardness of water and salt in the dishwasher
The main problem of hard water is that calcium and magnesium ions bind surfactants, greatly reducing its effectiveness. Two methods are used to combat stiffness: water treatment and additional components in a detergent. In the dishwasher, ion exchange resin provides water treatment. The resin captures Ca 2+ and Mg 2+ , and in exchange yields Na + . Over time, the resin “clogs” and needs to be regenerated. For this, use a brine from NaCl. As a result of a discussion in a previous post, Genegineer made a good point about the source of salt.

Extra without iodine and fluoride, of course, is completely suitable, but there is a more convenient option - pressed salt tablets for industrial plants. Sold a lot where, in particular in Leroy Merlin. About 600 rubles per 25 kg.
One more photo

Powder additives are the second way. One way or another, they bind the problem ions and remove them from the solution. For example, the same EDTA forms strong complexes with them and does not react with surfactants. At the same time, by the way, it “cleans” salt deposits on heating elements and rust, which cannot be there. And heavy metals binds. Useful substance. Polyphosphates, polycarboxylates and others serve for the same purpose. And in our version all the same sodium carbonate will work. The simpler the better. The reaction is simple to disgrace: Ca 2+ + Na 2 CO 3 = CaCO 3 ↓ + 2Na + . That is, those milligrams of calcium salts that are in solution instantly precipitate in the form of chalk. With magnesium is similar.
As tbl correctly noted :
The "ion exchange filter regulator" controls how often the resin needs to be regenerated. The water used for washing dishes still passes through this resin, leaving magnesium and calcium ions there. As a result, even if washing uses substances that lower the hardness of water, the resin will still require regeneration. Therefore, the regulator must be put in the position that corresponds to the hardness of the water. And add salt as needed to the regeneration container. Unless, of course, you want to completely remain without an ion-exchange filter.
Sudo checkinstall

Putting all the components together. We are already smart, we know why each of them is needed. At the same time we cheapen the final product to a minimum.
The basis is calcium carbonate. The cheapest component. In the last post, for lack of washing soda, I baked baking soda to get carbonate. It can be easier. It’s easier to buy granular washing soda right away. I took in Komus, which, as it turned out, sells not only office supplies, but also a bunch of everything, including industrial chemistry. Here is a link to it . Un granulated cakes and dusts.
Oxidizing agent- oxygen bleach in the form of sodium percarbonate and TAED for activation. Since it turns into the same washing soda during decay, you can use it as a base. It is all evil powder. But it is twice as expensive, albeit insignificant in absolute terms. Washing soda - 49 rubles per kg, percarbonate with activator - 100 rubles per kg.
Surfactant- an additional component. It is important not to foam too much. The pump is designed for incompressible fluid and may overheat when pumping foam. And foam can fill the electronics if you're out of luck. As a source of surfactants, you can use a regular powder for dishwashers, but as a small admixture to the main and cheap. Or, if you do not suffer from chemophobia, just take any powder for automatic washing machines without unnecessary impurities such as optical brightener.

I tested on the Biolan machine. Its composition: phosphonates (15% or more, but less than 30%), anionic surfactants (5% or more, but less than 15%), nonionic surfactants (less than 5%), enzymes, flavoring agent. Phosphonates are useful for softening water, the flavor is superfluous, but does not smell too much. But the enzymes will come to an end. Tender enzymes will be mercilessly consumed by active oxygen and alkali.
Test results
A new version of the powder with an oxidizing agent works better with protein contaminants. He also cleans organics from small pores cleaner due to foaming hydrogen peroxide. Often jets of water simply do not finish into the complex pockets of objects. For example, tricky parts from food processors.
Pictured are dishes before washing, contaminants in the form of buckwheat porridge and pizza dough with olive oil:


After washing:


Rinse aid
Dear amarao was upset by the fact that there was no prescription for the rinse aid:
But you did not reproduce the rinse aid liquid, although all the most interesting know-how is hidden there.
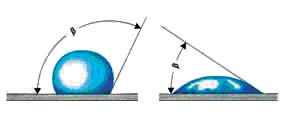
Rinse aid is needed in order to reduce surface tension and increase the area of drops. As a result, a large drop surface accelerates drying and salts from tap water are distributed over a larger area. In fact, stains are simply masked. A little surfactant remains on the dishes, which is uncritical.
The composition of most rinses: surfactants (reducing surface tension), citric acid (to improve the solubility of salts), isopropyl alcohol (antifoam). As a surfactant, it is most logical to take a detergent for a hand wash. It is designed for crooked users who do not wash it off dishes well. For example, abstract Fairy. You can take ethyl alcohol, although it’s easier to find. You can not take it at all, but then there is a chance to get excess foam.
Recipes
Rinse aid:
Citric acid - 5 g.
Isopropyl alcohol - 100 g.
Detergent for dishes - 0.05-0.1 ml (two drops, but better measured with an insulin syringe)
Water - up to 1 liter
Powder for dishwasher (cheaper option):
Washing soda granular - 50%
Sodium percarbonate + TAED - 30%
Washing powder - 20%
Dishwashing powder (evil version):
Sodium percarbonate + TAED - 80%
Washing powder - 20%
What can be improved? It would be nice to add EDTA separately. I described its properties above. It is in the composition of the powder, but not much, given the dilution. And it is sold only in bags, as a rule. Not very expensive, but the price in the region of 5000 per bag is too much. You can get it at the departments of chemistry from universities, you can get it in boiler rooms. Trilon-B is the same commercial name.
Then the powder recipe will be as follows:
Granular washing soda - 40%
Sodium percarbonate + TAED - 30%
EDTA - 10%
Washing powder - 20%
Warning
Please do not wash your grandmother's antique set, hand-forged Santoka knife and other Faberge eggs in the dishwasher. And do not eat powder. And use it in another way inside. It is alkaline, and even with peroxide. Thanks. Conventional powders are no better, but at least I clearly warn you about this.
Update Found another great component

In the same FixPrice from the same Shchelkovo Chemical Plant, he found an excellent mixture of sodium carbonate, sodium polyphosphate and EDTA (Trilon-B). No extra surfactants or anything else. The formal purpose is to carefully descale the heater. And just with the complexing agent as I wanted. Sodium polyphosphate - additionally softens water by binding hardness salts, and at the same time it is a weak surfactant. Theoretically, you can do with a mixture of oxygen bleach of the same company with this option. This will result in an alkaline environment, the complexing agent will improve the washing and the heating elements will protect, the polyphosphate will be washed additionally.
The price is the same cheap - 49 rubles for 750 grams.
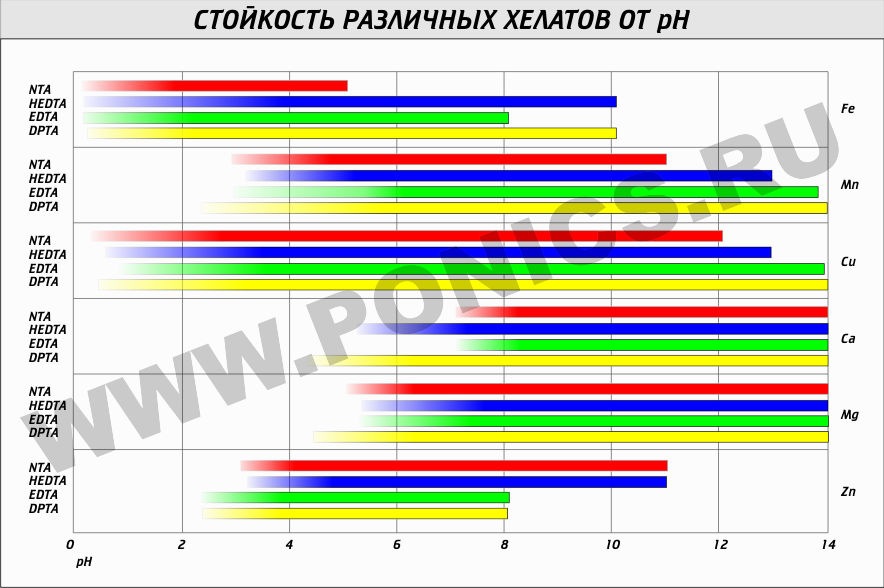
Table of ion binding by complexing agents depending on pH, our EDTA is green:
Who will test - try this recipe too, if you can buy it. Too lazy to count on the components, I will give an approximate option.
New recipe:
30% Oxygen bleach (percarbonate> 30%, sodium carbonate> 30%, TAED <5%)
60% Refine from scale (sodium carbonate> 30%, sodium tripolyphosphate 5-15%, Trilon-B <5% )
10% Washing powder / dishwashing powder (surfactant and small additives)
Update 2. In the process of testing, corrosion of stainless steel was observed on a knife for chopping meat and one of the pots.
Recipe recalled from everyday use. The combination of oxygen with an alkaline medium still cleans too well. The solution turned out to be more aggressive than planned. The dishwasher itself did not work, but I decided to change the recipe. Pure soda did not give such problems for several months of use, and the pH of regular dishwashing detergents is comparable. For now, please use at your own risk. Refine from scale (sodium carbonate> 30%, sodium tripolyphosphate 5-15%, Trilon-B <5%) goes into the main direction of the tests.

