Biomarkers of aging. Frailty panel. Part 1
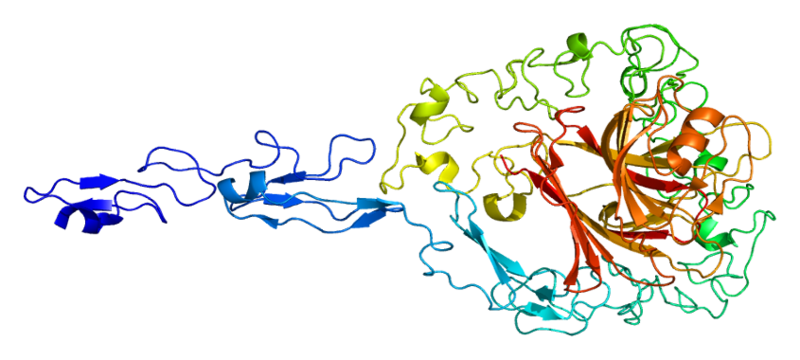
The structure of the protein Trombospondin-2
At the end of July of this year, a large study of an international group of researchers devoted to the search for effective biomarkers of aging in the framework of the concept of frailty (fragility or senile asthenia) was published .
The term “frailty” itself was introduced into the scientific circulation of the JH Friend back in 1954, having first appeared in the article Science “Alas for Human Frailties!” . The very concept of frailty today is defined as the presence of the following signs in an elderly person: weight loss (sarcopenia), dynamometric reduction in hand strength, pronounced weakness and increased fatigue, reduced movement speed, and a significant decrease in physical activity. It is believed that frailty(senile asthenia) occurs when there are three or more symptoms, but if there are one or two symptoms, then this is senile disorder.
The Federal Council on Aging of the United States of America coined the term “frailty” for a special group of older people with “significant physical, cognitive, and emotional disabilities that need extra attention . ” According to modern estimates, the number of older people who can be classified as frailty is 12.9% today, senile is 48.9%, which, in the absence of adequate treatment and rehabilitation measures, turns into expanded form within 4–5 years.
The authors of this study determined frailty as the "main phenotype of accelerated aging describing multiorgan dysfunction or multimorbid (i.e. the presence of two more chronic diseases and etiologically and pathogenetically unconnected) with increased vulnerability to additional diseases in elderly people" .
Scientists used gene expression databases (http://genomics.senescence.info/genes, including GenAge, AnAge, LongevityMap, CellAge, DrugAge, Digital Aging Atlas) to identify genes that are regulated during aging, longevity, and age-related diseases. In doing so, they paid particular attention to secreted factors and molecules found in biological fluids as potential biomarkers.
At the first stage of work, factors that were widely expressed and associated with several paths of “distinguishing signs of aging” were identified, as well as those already used as biomarkers in the diagnosis of age-related pathologies. Then this set of biomarkers was expanded in accordance with the experience of the authors of the study.
The search for biomarkers was conducted in six directions, six "signs of aging":
1. Inflammation
2. Mitochondria and apoptosis
3. Calcium
homeostasis , 4. Fibrosis
5. NMJ (neuromuscular junction) and neurons
6. Cytoskeleton and hormones.
44 potential biomarkers were analyzed, of which 19 received a high priority score, 22 were identified as medium priority.
As a result, the researchers identified a main and extended panel of biomarkers frailty , consisting of six points.
1. Inflammation. General changes in the immune system, affecting both adaptive and innate immune responses, were one of the most important "signs of aging," and immunological factors were among the first markers described for senile asthenia. The aging process is closely related to the systemic increase in proinflammatory mediators of various nature. This increase may be either directly related to the sustained exposure of infectious agents throughout life, or age-related changes in the intestinal microbiota.
The reason for this may be metabolic dysfunction observed in obesity, as well as the secretion of antigens caused by cell death and the subsequent accumulation of cellular debris. In general, inflammation leads to chronic stimulation of immune cells, which transform into low-grade and long-lasting inflammation, which affects both congenital and adaptive immune responses.
In addition, aging leads to noticeable changes in the phenotypes and functions of immune cells. In general, this phenotype, called “immunogenicity,” contributes to the accumulation of cellular and molecular damage in aging tissues, potentiates many age disorders (for example, atherosclerosis, diabetes and neurodegenerative diseases) and, most importantly, reduces the effective response to infections, cancer and other tissue damage. .
The following biomarkers were identified due to inflammation:
- CD14 antigen . It is a glycoprotein that is expressed mainly by monocytes and macrophages. As part of the complex TLR 4 - CD14, it participates in the body's immune response - the binding of bacterial lipopolysaccharides.
- Fractalkine (CX3CL1) . Fractalkine is produced most of all in activated endothelial cells, smooth muscle cells and macrophages. It enhances the migration of leukocytes from the bloodstream to the tissue by increasing selectin-mediated binding, causing adhesion and, ultimately, migration of leukocytes through the endothelial layer. CX3CL1 also has anti-apoptotic properties.
- Pentraxin (PTX3) . Pentraxin acts as a component of humoral innate immunity and is induced by various inflammatory cytokines in peripheral blood leukocytes and myeloid dendritic cells.
Pentraxin is also secreted by endothelial cells, vascular smooth muscle cells, fibroblasts and adipocytes, contributing to the differentiation of fibrocytes and playing a role in angiogenesis and tissue remodeling. - Adhesion molecules sVCAM / sICAM (intercellular adhesion molecule of the 1st type, adhesion molecule of the vascular endothelium of the 1st type). They belong to the immunoglobulin superfamily. ICAM-1 is expressed on epithelial and dendritic cells, fibroblasts, tissue macrophages, and VCAM-1 is expressed on tissue macrophages, dendritic cells, bone fibroblasts, myoblasts, and muscle fibers.
- Interleukin 6 (IL-6) . It is one of the most important mediators of the acute phase of inflammation. It is expressed in various tissues, including skeletal muscle, bladder, gallbladder, appendix, esophagus, bone marrow, lungs, adrenal glands, prostate, and adipose tissue. IL-6 is mainly produced in areas of inflammation and induces a transcriptional inflammatory response through IL-6RA (IL-6 receptor - alpha).
- Interferon γ is an inducible protein (IP-10) , another designation is CXCL10 CXC motif chemokine 10. It is an IFN-induced chemokine of the CXC subfamily and a ligand for the CXCR3 receptor, which is mainly expressed by activated T-lymphocytes and fibroblasts.
The binding of CXCL10 to CXCR3 leads to the migration of T cells, to the stimulation of monocytes and NK cells, as well as to modulation of the expression of the adhesion molecule and induction of apoptosis
2. Mitochondria and apoptosis . Mitochondria play a central role in the production of ATP, as well as in reducing the basal metabolic rate and physical performance. Age-related dysfunction of mitochondria includes a decrease in the rate of electron transfer, increased permeability to the H + inner membrane, and impaired ATP synthesis.
In addition, mitochondrial mutations of DNA accumulate in the process of aging. All cells, especially neurons and muscle cells, are very sensitive to mitochondrial dysfunction associated with oxidative damage, since large amounts of ATP are needed to maintain neuronal processes and contractile function.
The following biomarkers were distinguished in connection with mitotic dysfunction:
- Growth differentiation factor -15 (growth differentiation factor 15, GDF15). Pleiotropic cytokine, a protein from the superfamily of transforming growth factor-beta. Involved in the inflammatory response and regulation of apoptosis during damage and during various pathological processes.
- Irisin (fibronectin type III domain containing 5, FNDC5). Membrane protein, which is a precursor of the peptide hormone irisin. It positively regulates the differentiation of brown fat, promotes mitochondrial biogenesis, maintains the function of mitochondria under hypoxic conditions, protects against apoptosis, and has anti-inflammatory activity.
- Wimentin (VIM) . A protein that plays an important role in maintaining cell integrity. Involved in the processes of apoptosis.
- Calcium homeostasis . Calcium plays an important role in many physiological and pathophysiological processes, extracellularly and intracellularly. Physiological calcium levels are fairly narrow, and even small changes can lead to huge dysfunctions.
Calcium is absorbed in the intestines, excreted from the body through the kidneys and its levels are mainly regulated by parathyroid hormones. Calcium is mainly found in the muscles, heart and bones. Within the cell, the endoplasmic reticulum (ER) and mitochondria are the primary storage and, under homeostatic conditions, relatively low calcium concentrations are present in the cytoplasm.
Cell viability and activity of a large number of enzymes depend on calcium. Thus, it is not surprising that disturbances in calcium homeostasis are associated with organ dysfunctions, old age, and many diseases.
The following biomarkers were highlighted here:
- S100 is calcium binding protein B (S100 calcium binding protein B, S100B). Belongs to the group of calcium-binding proteins S100, is produced in glial cells, mainly astrocytes, as well as in adipocytes. Participates in intracellular and extracellular calcium signal transduction. S100B is involved in the regulation of a number of cellular processes, such as cell cycle progression and differentiation.
- Regucalcin (Regucalcin, RGN) . Also known as aging protein-30 (senescence marker protein-30, SMP30). Predominantly expressed in the liver and kidneys. Plays an important role in calcium homeostasis, is involved in calcium-induced oxidative stress.
- Calreticulin (Calreticulin, CALR) . Multifunctional protein that binds Ca 2+ ions. It acts in mitochondria, on the surface of proapoptotic cells and in the endoplasmic reticulum, where it binds to incorrectly folded proteins to prevent their export. It has a number of functions in apoptosis and in the immune response.
- Fibrosis . Fibrosis is the process of formation of fibrous tissue, which can be part of the normal process of healing wounds after injury. At the same time, fibrous tissue can also permanently replace functional tissues as a result of aging.
As a result, fibrous tissues accumulate in organs such as the heart, lungs, kidneys, liver, and prevent the normal function of organs. This leads to hyperproliferation and increased inflammation due to the presence of various inflammatory cells (neutrophils and macrophages).
In addition, uncontrolled protease activity interferes with the normal repair mechanisms, and this can lead to an increase in fibrous tissue. Many cytokines, such as IL-13 (interleukin 13), IL-21 (interleukin-21), TGF-beta, and chemokines, such as MCP-1 and MIP-1 β, are involved in fibrosis.
In addition, angiogenic factors (eg, VEGF (vascular endothelial growth factor)), growth factors (eg, PDGF (platelet growth factor)) and components of the renin-angiotensin-aldosterone system have been identified as important regulators of fibrosis and are being explored as potential targets. anti-fibrous drugs.
The following biomarkers were identified for fibrosis:
- Transforming growth factor beta (TGF-beta) . Protein that controls proliferation, cell differentiation and other functions in most cells. The representative of cytokines, is involved in the immune response, cancer, cardiovascular diseases, diabetes and many other pathologies.
- Plasminogen activator inhibitor 1 (PAI-1 or Serpine E1). Inhibits the work of tissue plasminogen activator and urokinase, which in turn activates the transfer of plasminogen to plasmin that cleaves fibrin of blood clots. Thus, PAI-1 adversely affects fibrinolysis and prevents the dissolution of blood clots, which increases the risk of vascular complications, various thromboembolism.
- Urokinase plasminogen activator ( urokinase plasminogen activator, uPA). It is a secreted serine protease that converts plasminogen to plasmin. Functionally associated with the plasminogen activator inhibitor described above 1. Activation of plasmin causes a proteolytic cascade that, depending on the physiological environment, is involved in thrombolysis or degradation of the extracellular matrix. This cascade is involved in vascular disease and cancer progression.
- Matrilysin, matrix metalloproteinase-7 (MMP-7) . MMP-7 is expressed in various organs and tissues, including the liver, lungs, heart, breast, spleen, brain, spinal cord, and pituitary gland. The functions of MMP-7 are closely related to processes such as morphogenesis, angiogenesis, and tissue repair. Its dysregulation is associated with fibrosis, the production of inflammatory cytokines and endocrine imbalances. Involved in a number of pathologies, such as cirrhosis of the liver, rheumatoid arthritis and cancer.
- Transglutaminase 2 (TGM2) . It is the most common member of the transglutaminase family and is expressed to varying degrees in almost all cell types. It plays a modulating role in the development of the nervous system, as well as a regulatory effect on neuronal cell death. Involved in various pathophysiological processes, such as wound healing, cell growth and survival, apoptosis and autophagy.
- Thrombospondin-2 (THBS2) . Part of the thrombospondin protein family is present in various tissues, such as epithelium and endothelium, connective tissue. It activates latent TGF-beta and plays an important role in the regulation of cell proliferation, apoptosis and angiogenesis.
- Angiotensinogen (angiotensinogen, AGT). The precursor of angiotensin is produced in the liver and is converted into angiotensin I by renin. Angiotensinogen levels are increased by plasma corticosteroids, estrogen, thyroid hormone and angiotensin II. It plays a key role in the regulation of systemic blood pressure, vasoconstriction, water consumption and sodium retention, in pro-inflammatory, prothrombotic and profibroznyh processes.
Angiotensin I and II
Prepared by: Alexey Rzheshevsky
