Battery that breathes
IBM continues to look to the future and work on projects that will revolutionize our lifestyle in the very near future. The Battery 500 , a project of a "breathing" battery, on which an average electric car can travel up to 500 miles (800 km) without recharging, refers to such projects . In the blue giant, they were able to design such a battery that generates electricity by “breathing” oxygen, and recharges - “exhaling” it into the atmosphere. Thanks to such a scheme (air exchange inside the battery), IBM researchers hope to reduce the size of the batteries, their weight, as well as indicators of efficiency - compared to ion batteries, the Battery 500 promises to be much more economical and efficient.
Scientists have long been looking toward lithium-air batteries, but so far only IBM has been able to build a working prototype. “The fundamental processes taking place in the battery are no longer in doubt,” says Winfred Wilke, who is responsible for this area (energy) at the company. IBM believes that with this technology it is really possible to build a car rechargeable battery, on a single charge of which it will be possible to travel up to 500 miles. Nevertheless, as Wickle notes, the prototype has a long way to go before it actually appears on the market: “There is a lot to do before we can install the battery in a real car.” But the blue giant expects that after 2020 the battery will be much more real than it is now, in terms of mass production and use.
At the moment, the following situation has developed in the automotive market - electric cars are still impractical, partly because of the limited resource (cars that are produced today can travel an average of 200-400 kilometers without recharging), partly because of the bulky batteries themselves. The ratio of the weight of the battery to the amount of energy that it can provide to the engine still does not reach the same ratio for classic ICEs, where on one tank you can make a trip with a distance of more than a thousand kilometers (or even more). Improvements introduced in battery technology today only slightly increase the amount of energy, but significantly increase the overall design.

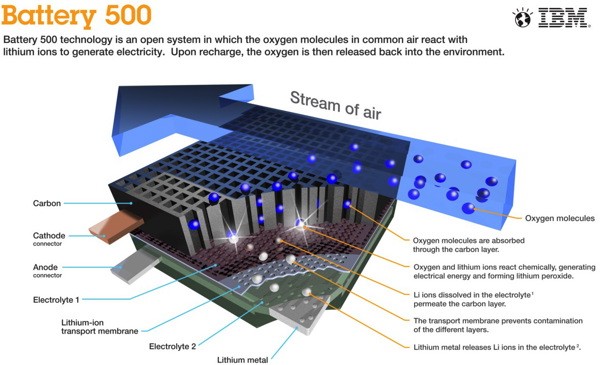
To describe in simple words what Wilka and his team managed to achieve is quite easy: they deprived the battery of the internal oxygen exchange necessary for the normal course of reactions in the battery, instead relying on oxygen, abundant in the atmosphere. The life-giving gas enters the cell of the "open system" in much the same way as the combustion chamber of a classic engine. Inside this cell, oxygen enters a very small space a little more than an angstrom (1.0 × 10 -10 meters), reacting there with lithium ions and electrons at the cathode of the battery. The reaction transforms lithium ions into lithium peroxide, releasing electrons and generating electricity for the engine.
“The main plus is that we no longer need to try to compress the reaction,” says Wilke: “A battery can produce up to 10,000 milliampere-hours per gram of cathode material used.”
At the same time, IBM immediately emphasized that such a large increase in efficiency would not result in a proportional increase in power at the moment when the first electric cars with this type of battery appeared on the market - it still uses the materials necessary to support the reaction and at the same time, “eating up” part of the generated energy. But the experiment itself still shows how much more energy can be generated / stored using the technology used.
As soon as the battery is saturated with oxygen, it reaches its maximum charge and must be connected to a power source for recharging, during which the battery just “exhales” the accumulated oxygen, returning lithium to its ionized state.
The research team led by Wilke, along with other teams of physicists from Zurich, used the IBM Blue Gene when designing the battery to model atomic processes, more specifically, understanding how the ions and molecules of the material the battery is made of will interact and what processes will occur .
In the near future, the team hopes to prepare a scientific article on the new technology, up to this point only some details will be available, most of which are described here. However, it can immediately be noted that recently discovered materials (such as graphene) are unlikely to be used in further experiments, and even more so in the production of lithium-air batteries - as Wilke says, everything related to carbon is not suitable for creating fuel cells, as they become unstable with prolonged use.
Introductory video in English:
