Ask Ethan # 93: The Random Newton Apple
- Transfer
If you combine all the random movements of internal molecules, how far and how quickly will the subject move?
Millions saw apples fall, and only Newton asked why.
- Bernard Baruch
One of the greatest pleasures of a scientist who writes on favorite topics for all comers is that from time to time you come across a person who has been interested in a question all his life, to which he has not received an answer. If you have such a feeling, you can send me your question, and you may be lucky just like Mike, who asks:
This question has been bothering me since childhood. If all the random thermal movement of molecules in an apple takes the same direction, how far will the apple move? And then what will happen?
If you think about the microscopic levels of large objects, what will you imagine?
Painted Apple Cells
Unpainted apple cells
Perhaps you imagine a cellular level increased hundreds of times more than we can see in a microscope. But you can go even deeper.
Each cell consists of organelles, each organelle has its own unique set of configurations of molecules, giving it structure and function, and each molecule itself consists of smaller particles: atoms, electrons, nuclei, and even smaller ones, quarks and gluons.
Perhaps you will imagine the smallest of particles of matter, and think about how they move there in the apple.

If this were an exact image of an apple, then to answer the question Mike would need to measure the temperature of the apple - say, it will be room temperature at 298 K - calculate the mass of particles, for example, the sugar molecule at 342.3 a. e. m., and use the kinetic molecular theory to find out how fast molecules are moving on average with this.
Get something like 147 m / s, or 529 km / h. This is three times faster than an apple flying out of an apple cannon.

If you manage to catch all the thermal energy of the movements of these atoms in an apple, and with 100% efficiency to translate it into the kinetic energy of an apple, then it will come out that way.
But with such reasoning, there are two problems, or rather, two reasons why an apple will never throw such a thing out.
1) An annoying law of conservation of momentum. Thermal motion is random, and therefore, for each atom or molecule moving in one direction, there is another atom or molecule moving in the opposite direction. Of course, individual components can move quickly, but on average, the apple's momentum is zero. Similarly, an apple can consist of 10 27 protons and 10 27electrons, but on average, no giant electric forces are observed, since its total charge is balanced and zero. For the same reason, it is impossible to take a random configuration of energy and turn it into a directional kinetic without some kind of compensation, and without an equal and oppositely directed impulse moving in the direction opposite to the direction of movement of the apple.
If this were the only limitation, it could be tricked.
One could send a small part of the mass of an apple in one direction with the help of a rebound: a small mass bounces off a large one, which bounces off an even larger one, and so on.

This method, by the way, is very important for nuclear physics, and works in a phenomenon known as the Mössbauer effect or nuclear gamma resonance. It immobilizes the nuclei of crystals, which leads to a small change in the momentum of the entire crystal, which causes individual particles (photons) to radiate with enormous energies / speeds. The reverse Mössbauer effect could allow the apple to fly relatively slowly (147 m / s), while a small part of it would fly in a different direction with a huge impulse.
But there is a second reason why this will not happen, and it is very weighty.
2) These atoms are not free, but bound into molecules, which are basically bound together into large solid structures. We used to imagine atoms bouncing off of each other - and it describes well liquids, and even better - gases and plasma. But we cannot apply the same approach to solid bodies. We get a vibration, rotational movement, but not free and fast kinetic.
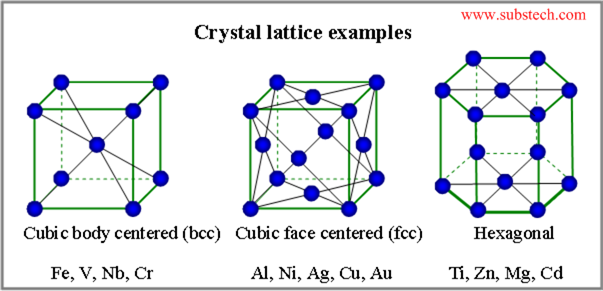
A large amount of energy is stored in the bonds of a solid, but the thermal energy present, which makes the atoms vibrate, is not enough to break these bonds, and therefore the apple remains in a solid state.
To break these bonds, you need a huge amount of thermal energy, which you cannot achieve unless you dry the apple, because the temperature above 373 K will simply boil all the water inside.

If we understand that there are no separate, free molecules of water, sugar and other molecules in our apple, but only large structures (like cells), we will find that individual “random” movements are actually much smaller. Even if we assume (and this would be a huge exaggeration) that the apple is divided into freely moving particles of a mass in nanograms, we find that their thermal motion is extremely small: the speeds are about 100 microns per second.
In other words, since the apple is solid and the molecules inside it are connected, this thermal movement will not allow you to achieve a noticeable speed. Even if you try to achieve this state, as a result you will get a warm apple that does not move anywhere.

And although this may not be the answer you have been waiting for, the consideration of the laws of physics helps us study the nature of matter and learn a little more about how the Universe works. Send me your questions and suggestions for the following articles.
