Anti-Aging: Senolithics and Stem Cell Therapy
In this article, we will try to consider two mechanisms of aging and approaches to their therapy: senescent cells (also called decrepit) and their destruction, as well as stem cells and their restoration and replenishment. Perhaps the fight against decrepit cells and the increase in the stem cell pool are complementary approaches that could give a cumulative result. In addition, such therapies are now at the forefront of research, some are already being applied in hospitals, the majority will go into practice in the coming years.
In one of the previous reviews, we talked in detail about the senescent cells and how scientists discovered these cells for the weak. But in short, these cells appear after damage, in the event that the cell could not heal the damage, and at the same time could not start the process of apoptosis and destroy itself.
A decrepit cell cannot divide, performs its functions poorly, and the most unpleasant thing is that it secretes signaling substances that help the surrounding cells to become decrepit. Today, the best approach to blocking this mechanism of aging is considered to be the killing of senescent cells, because even in the old organism there are few of them, and no important functions will be lost. For the removal of such cells, special preparations were developed, called "senolitics" (from senile - decrepit and lytic - destructive).
This research began in 2011. Then James Kirkland and his colleagues conducted an experiment surprising in its complexity and elegance. They have developed a line of specially modified transgenic mice INK-ATTAC (INK-linked apoptosis through targeted activation of Caspase).
The cells of these rodents were subject to apoptosis, cell suicide, associated with p16 Ink4a protein and targeted activation of caspase. Protein p16 Ink4a shows its activity in decrepit cells, blocking the ability of cells to divide. And because apoptosis had a targeted "senoliticheskuyu" action, without affecting normal cells. Activation of caspase initiating apoptosis occurred after administration of the special drug AP20187 to mice.
The results of this workshowed that removing senescent cells from the body delayed the development of age-related pathologies: “ In adipose tissue, skeletal muscles and eye cells, in which p16 Ink4a contributes to the acquisition of age-related pathologies, the removal of p16 (Ink4a) -expressing cells delayed the onset of pathological processes. In addition, the delay at the end of life weakened the progression of already established age-related disorders. These data indicate that cellular aging is causally related to the occurrence of age-related pathologies, and that removing aging cells can prevent or slow down tissue dysfunction and prolong health. »

James Kirkland MD, PhD
After that, James Kirkland became a real leader in the study of Senolithic and in fact monopolized this area of research for the next five years, constantly publishing in the best journals of the world all new molecules with senolytic activity.
In 2014, scientists described one of the mechanisms that regulates cellular aging and can be used in the therapy of senescent. The focus of their work was the DBC1 protein (Deleted in Breast Cancer 1), which regulates several nuclear proteins, including the well-known gene regulator of aging SIRT1. It changes activity in various tissues: with a decrease in DBC1 expression, SIRT1 activity increases and vice versa. It is known that a diet with a high fat content enhances the expression of DBC1, reducing the expression of SIRT1 in the liver and leading to its defeat, steatohepatitis.
In addition, it is known that SIRT1 proteins can suppress cellular aging. Preadipocytes of mice with knockout of the DBC1 gene after 12 weeks of feeding with a high fat content showed fewer signs of cellular aging . They had lower levels of p16 Ink4a involved in stopping the cycle, as well as markers of the secretory phenotype associated with aging (SASP): MCP-1, TNF-α and IL-6. These substances are the very same set of harmful molecules that cause all nearby cells to age.
In addition, a smaller amount of γ-H2AX, a known marker of DNA damage, was detected in preadipocytes.

The mechanism of interaction between the DBC1 and SIRT1 genes, and that is what therapy is aimed for.
In 2017, an article was published in the best scientific journal ScienceHarvard scientists describing a method for suppressing DBC1 by administering nicotinamide mononucleotide (NMN) to mice. In addition, it was found that the suppression of DBC1 also has a beneficial effect on the activation of the DNA repair system after injury. By the way, in its natural form, the NMN enzyme, which was injected into mice, is contained in broccoli.
It is important to understand that in some cases the transfer of a cell to a state of decrepitude, which makes DBC1 protein, can prevent the cell from degenerating into a tumor. Therefore, scientists continued to focus the main focus of research on the suppression of the p16 protein (Ink4a).
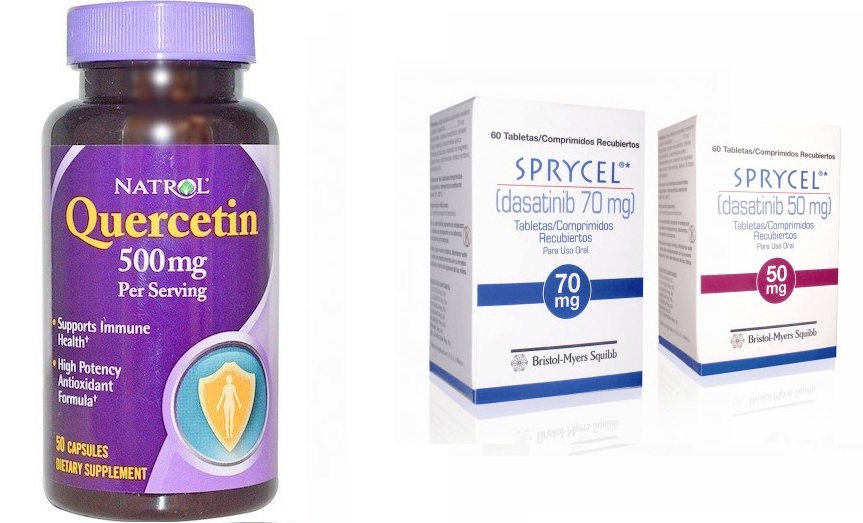
It was for killing cells with this marker that the first senolitic drug was discovered. It was a combination of two substances: dasatinib, an anticancer agent with directional action, and quercetin - a flavonoid with antioxidant and anti-inflammatory properties.
In the course of the study, scientists tested 46 potential drugs aimed at activating cellular suicide, apoptosis, in aging cells. As a result, dasatinib and quercetin showed the best result.
First, a combination of these drugs (dasatinib 5 mg / kg and quercetin 50 mg / kg) was given orally to older mice (24 months old).
After 5 days, preadipocytes and endothelial cells showed a decrease in the levels of cell aging markers - a special form of beta-galactosidase (SA-βgal) and mRNA of the p16 gene. In addition, there was an improvement in cardiovascular and physical function in older mice.
The scientists then tested the effects of dasatinib and quercetin on a mouse model of accelerated aging. Introduction of senolithic to such mice resulted in a decrease in the expression of aging markers in several tissues, a decrease and weakening of the signs of aging were observed in general: spinal curvature decreased, tremor, urinary incontinence, gait disturbance, hind limb paralysis, etc. The healthy period of life increased.
Dasatinib and quercetin also removed senescent cells from irradiated tissues.
All the data obtained allowed to state that “senolithics can be used in the future to prevent cardiovascular diseases, senile asthenia, as well as slow recovery or dysfunction after chemotherapy or radiation, neurodegenerative disorders, osteoporosis, osteoarthritis, other diseases of bones and joints and adverse pathologies related to chronological aging »
Follow-up researchconfirmed and expanded the data obtained on the senolytic properties of dasatinib and quercetin. Rodent treatment with dasatinib and quercetin improved the ejection fraction of the heart and increased vascular reactivity in old mice after one 3-day course of treatment. Also, their introduction reduced vascular calcification and improved the condition of the vessels with a high-fat, hypercholesterolemic diet.
Dasatinib and quercetin improved pulmonary function and decreased pulmonary fibrosis in a mouse model of idiopathic pulmonary fibrosis, reduced liver steatosis caused by a high-fat diet, and reduced osteoporosis in old mice.
In a mouse model of human progeroid syndrome, dasatinib and quercetin reduced brittleness, osteoporosis, loss of glycosaminoglycans in the intervertebral discs, and spondylosis.
Recently, in January of this year, the results of the first open pilot study of senoliths in humans were published . The combination of dasatinib and quercetin improved physical performance in elderly people with idiopathic pulmonary fibrosis.
Navitoclax
In 2016, Kirkland and colleagues presented another potential senolithic drug, navitoclax (ABT263). Navitolax is an inhibitor of proteins of the Bcl-2 family, which are involved in the regulation of apoptosis, and is undergoing clinical trials as an antitumor drug. It revealedthat navitolax, inhibiting the activity of Bcl proteins, stimulated apoptosis in some old cells. It acted as a senolithic on human umbilical vein endothelial cells (HUVEC), on human lung fibroblasts (IMR90) and mouse embryonic fibroblasts (MEF), but had no senolytic effect on human preadipocytes.
In the same year, another group of scientists described the effect of navitolax in experiments with rodents that normally aged and were exposed to radiation. Oral administration of navitolax to irradiated and aging mice effectively removed senescent cells, including aging bone marrow hematopoietic stem cells and muscle stem cells. The authors made this conclusion from their study: “Our results show that the selective purification of senescent cells using a pharmacological agent is partly due to the rejuvenation of old stem cells of various tissues. Thus, senolithic drugs can be a new class of anti-aging and anti-radiation agents . ”
In 2017, the ability of navitolax to influence the development of fibrosis was demonstrated . Navitolax removed apoptosis-resistant myofibroblasts that participated in the formation of fibrosis in a mouse model of scleroderma (an autoimmune disease characterized by multiorgan fibrosis).
For the last three years, the senolitic effect of tocotrienols, chemicals from the vitamin E family has been described in detail. For a long time, tocotrienols were in the shadow of other chemicals of the same family, tocopherols, and their active study began quite recently. As it turned out, tocotrienols have great potential in the fight against various diseases and aging. They have a pronounced antioxidant and neuroprotective activity.
Their ability to exert a protective effect on neurons and alleviate the symptoms of Parkinson’s disease has been shown. Also tocotrienolscan help prevent bone loss due to osteoporosis and the onset of stomach ulcers and gastritis due to stress. In addition, tocotrienols had a prophylactic effect on the development of cardiovascular pathologies and had antitumor properties, stimulating apoptosis in cancer cells.
This last property, induction of apoptosis, allowed scientists to view tocotrienols as potential senolitics. Why it was conducted a number of studies , showing them senoliticheskuyu efficiency . Based on all of the above, it does not seem at all accidental that in the title of one of the scientific articles, tocotrienols are designated as “vitamin of the 21st century” with largeclinical capabilities .
Perhaps it would not be a great exaggeration to say that tocotrienols possess one of the most significant potentials as senolytic agents.
Another class of senolithic, found by James Kirkland and his colleagues, were inhibitors of the protein Hsp90 (Heat shock protein 90). Hsp90 belongs to the heat shock protein family. They perform the function of chaperones in the body (from the French shaperon - nanny), participating in folding (folding into the correct structure), degradation and stabilization of the protein, correcting errors in the protein structure.
But, as is often the case, Hsp90 has a dual role in the body: useful and not very. In addition to protecting vital proteins, Hsp90 supports proteins involved in carcinogenesis - it stabilizes several unstable oncogenic factors, such as mutant EGFR, BRAF and HER2, as well as some anti-apoptotic factors, preventing the removal of "damaged" cells.
Kickrland and colleagues found that inhibitors (i.e., activity suppressors) of the Hsp90 protein (geldanamycin, tanesimycin, 17-DMAG, etc.) also have senolytic properties. The mechanism by which Hsp90 inhibitors removed old cells included the effect on the activated form of AKT protein kinase, which suppresses apoptosis, affecting MTOR, NF-kB, Foxo3a, and other signaling pathways in cancer and old cells.
The introduction of transgenic mice with accelerated aging of one of the Hsp90 inhibitors, 17-DMAG, mitigated and eliminated the occurrence of several inherent age-related symptoms: kyphosis, dystonia, tremor, poor coat, ataxia, gait disorder, etc. According to the authors of the study, their results show that the HSP90 protein is a real molecular target for the destruction of senescent cells .
Finally, one of the latest discoveries on the “senolithic front” is the senolithic fizetin, presented in September this year by the same Kirkland and his team. Physetin is a famous flavonoid contained in many vegetables, fruits and berries (cucumbers, strawberries, persimmons, grapes, onions, etc.).
The objective of this study, according to the authors, was to identify another flavonoid with stronger senolitic properties than quercetin. A total of 10 flavonoids were analyzed. In senescent mouse embryonic fibroblasts and in human fibroblasts, fisetin most effectively lowered the levels of cell aging markers. Then he was examined on animals.
In the progeroid mouse model, administration of fizetin significantly reduced the expression of cell aging markers in all tissues. A decrease in the expression of p16 Ink4a, p21 Cip1 and SASP factors in peripheral blood CD3 + T cells, a cell type that demonstrates a steady increase in the expression of p16 INK4a in old people, was also observed. In addition, fizetin reduced oxidative stress in the liver, acting as an antioxidant and increasing levels of another antioxidant, intracellular glutathione.
In normally aging old mice, fizetin also reduced markers of cellular aging, but, most importantly, caused an increase in the life expectancy of rodents. The authors of the work describe their discovery with great enthusiasm: “Our results show that physetin is aimed at multiple, but not all types of aging cells in the body. In addition, by reducing the percentage of aging cells, Fizetin reduces the expression of aging markers in several organs. This leads to an improvement in tissue homeostasis and a reduction in multiple age-related pathologies, which is consistent with the impact on the fundamental aging process.
The fact that fizetin has reduced the proportion of aging T and NK cells can help strengthen the positive effects of physietin, since healthy immune cells are important for cleaning aging cells. Likewise, Physuetine reduces markers of inflammation and oxidative stress. ” Considering that physetin is a natural product found in food products and no adverse side effects have been found for it, the authors of the study suggest that physetin can soon be used in the treatment of humans .
And most recently , in November of this year, British scientists presented two more potential senolitik: antibiotics roxithromycin and azithromycin, which are aimed at aging human fibroblasts.
In the light of the foregoing, it seems quite logical that the elimination by senolithic cells of senescent cells in the elderly should improve health and increase longevity. Experiments with rodents modified INK-ATTAC (which we mentioned at the very beginning) showed that cleaning the body of a part of old cells by administering ap2014-activating drug AP20187 increased the life of mice compared to the control group by 17–35%. Decrease in markers of cellular aging was recorded immediately in several tissues: in the lungs, heart, kidneys, skeletal muscles, spleen. In addition, the function of the heart, kidneys and eyes remained normal in rodents , good physical activity and slowed tumor genesis.
In our opinion, after the treatment with senoliths, it seems quite logical to carry out the second stage, which helps to restore organs and tissues, to carry out replacement cell therapy with stem cells.
Stem cells used in cell therapy come in several forms. The most promising use of cell therapy may be in the treatment of age-related bone diseases, in pathologies that are poorly amenable to medical treatment (cardiovascular and neurodegenerative), as well as in the restoration of immune function. Consider briefly these three areas of cell therapy.
Mesenchymal stem cells (MSCs) are believed to have the greatest potential for tissue repair in cardiovascular diseases. MSCs were opened half a century ago by Soviet scientists, Alexander Friedenstein and his colleagues.
They have their own characteristic features: the ability to symmetric and asymmetric division, high proliferative potential and ability to adhere, fibroblast-like morphology, the formation of colonies in culture and easily induced differentiation. It is their last property, discovered not so long ago - the ability to differentiate into different types of cells - makes MSCs the most appropriate tool in cell therapy.
Another little-used type of stem cells is hematopoietic stem cells (HSC), which are involved in the formation of the immune system and blood formation and are only found in the bone marrow. Despite the fact that they are constantly divided in the bone marrow, there are relatively few of them, and they are rarely considered as an instrument for the treatment of aging. Their typical field of application is for bone marrow transplantation after surgery.
In the bone marrow, undifferentiated MSCs create a microenvironment for another type of bone marrow stem cells - hematopoietic stem cells, producing matrix molecules: fibronectin, laminin, collagen, etc. Also, MSCs secrete cytokines necessary for differentiation of HSCs. In cell therapy,
MSCs are used in three main areas:
Due to its great plasticity, MSCs can differentiate into a wide range of cells of various types: into striated and smooth muscle cells, into cardiomyocytes, into cells of bone and nervous tissue, liver, kidneys, pancreas, immune system cells and many others. In fact, we are talking about the whole reserve stem cell hospital in our bone marrow, ready to help in a wide variety of emergency situations. In experiments with animalsit was traced how the labeled MSCs introduced into the body were then detected in the cells of the brain, heart and other organs. A characteristic feature of MSCs is their predominant introduction into tissues with foci of damage, which demonstrates their regenerative potential. This is also true for the treatment of heart diseases, the cells of which, after injuries, do not have the ability to regenerate themselves.
If you take as an example of heart disease, then there are several criteria why MSCs are suitable for the treatment of heart attacks :
There are three main mechanisms of action of MSCs that underlie their beneficial effects on the heart:
Thus, already conducted clinical studies have shown that the introduction of MSCs to patients after myocardial infarction improved heart function (left ventricular function), improved physical condition and reduced mortality [22-24].
It is known that cardiac pathologies are accompanied by the death of cardiomyocytes. The introduction of MSCs is accompanied by a decrease in the apoptotic processes in the heart and scar reduction. Also, the work performed showed that MSCs play a large role in the processes of antiogenesis (the formation of new blood vessels), which are extremely important in the restoration of cardiac tissue.
In addition, MSC transplantation stimulated the proliferation and differentiation of endogenous heart stem cells, which are involved in the replacement of damaged tissue with a new one, capable of normal contraction. Over the past 15 years , a number of preclinical (in animal models) and clinical (in humans) have been carried out , showing the effectiveness of MSC in heart disease.
Another promising direction in the use of MSCs is the therapy of neurodegenerative pathologies, the medical treatment of most of which does not exist today. It has been established that MSCs can cross the blood-brain barrier and migrate to different areas of the brain.
The experimental studies described the participation of MSCs in the regeneration of damaged brain structures. Under the influence of microenvironment factors, introduced MSCs integrate into the damage area, form numerous contacts with neurons, acquire a neuronal phenotype (expression of nestin, a specific enolase, tyrosine hydroxylase, etc.) and functional activity of dopaminergic neurons. MSCs also have a neuroprotective effect, differentiating into microglial cells that provide trophic support for damaged tissues. MSCs activate the processes of neurogenesis by stimulating the neuronal stem cells of the subventricular zone of the brain, which then migrate, proliferate, differentiate and replace dead dopaminergic neurons.
According to scientists, today we can distinguish 3 main results of studies of cellular therapy for neurodegeneration:
Over the past 10 years, a number of preclinical studies in animal models have been carried out, showing a beneficial effect of cell therapy on neuropathology. Thus, a positive therapeutic effect was demonstrated from the intravenous administration of human MSCs isolated from adipose tissue to transgenic mice with Alzheimer's disease.
To check the migration of MSCs introduced into the brain, the researchers labeled them with fluorescent magnetic nanoparticles. Fluorescence signals from organs extracted 3 days after the transplantation of cells showed that the cells had spread to all organs, including the brain. After dissection of the brain into 5 separate areas (olfactory bulb, hippocampus, cerebellum, brain, midbrain and cortex), fluorescent signals of nanoparticles were detected in all areas of the brain, except for the olfactory bulb.
The positive effect was manifested in the improvement of cognitive abilities (learning and memory), the acceleration of endogenous neurogenesis and the increase in synaptic stability in the rodent brain. It was also recordedreducing the number of amyloid plaques in the brain, reducing the levels of beta-amyloid and the terminal fragment of amyloid precursor and reducing the apoptotic processes in the brain.
In another study, scientists injected MSCs from adipose tissue in mice with simulated amyotrophic lateral sclerosis. The outcome of the therapy was a slowdown in the development of movement disorders, an increase in the number of lumbar motoneurons and neuron growth factors.
StudyEffects of cell therapy on the treatment of Parkinson's disease was performed on rodents and monkeys. In rats with rotenone-induced Parkinson's disease, stem cell therapy caused immunomodulatory, anti-inflammatory and neurotrophic effects. Rodent serum levels of transforming growth factor β, monocyte chemoattractant protein and levels of dopamine in the brain increased. Therapy of MSCs from adipose tissue in combination with adenovirus containing NTN and tyrosine hydroxylase (TH) (Ad-NTN-TH), rhesus macaques with the Parkinson's disease model led to neuroprotective effects, reduced tremor and increased mobility.
These results are particularly important, since fatty tissue in significant quantities (up to 300 ml or more) can be obtained under local anesthesia with relatively little painful cosmetic liposuction, lipoaspiration of subcutaneous fat, or by excision of fat deposits. This tissue serves as a source of MSC for transplantation and tissue engineering.
In addition to the cardiovascular and neurodegenerative pathologies discussed above, cell therapy has great potential in restoring immune function, which, as is well known, worsens with age. For these purposes, another type of bone marrow stem cells is used - hematopoietic stem cells (HSC).
The immune system is closely intertwined with the nervous and endocrine systems, carrying out the regulation of all body processes, including making a huge contribution to the aging process. In addition, the deterioration of the functions of immunity with age makes a person poorly protected by various kinds of infections, worse purifies the body from old cells and can lead to the development of autoimmune and inflammatory processes. All this, naturally, shortens the life span and accelerates aging.
It is logical to assume that maintaining immunity is normal is one of the main strategies for combating aging. One of the mechanisms that can keep the immune system in good condition is bone marrow cell transplantation. The concept of this method is based on enhancing the aging immune system by young, healthy, autologous (ie, own) hematopoietic stem cells (HSC), which were collected from the same person in his youth and cryoconserved for a long period. Upon reaching old age, these own stem cells introduced into the body can rejuvenate the immune system and improve immune functions. This process is called autologous hematopoietic stem cell transplantation (haHSCT).
HSC transplantation itself has a half-century history and has been successfully used to restore immune function in the treatment of cancer and autoimmune diseases. About 40 thousand HSC transplantations are done every year in the world, the total number of which has reached 1 million over the entire period of the study. Studies have shown that HSC transplanted are included in the hematopoietic and immune functions of the recipient that were irradiated or chemically immunized during chemotherapy. The transplanted HSCs colonize the bone marrow, thereby establishing the complete set of cells necessary for the normal immune and hematological status of the patient.
At the same time, when a HSC is transplanted from an outside donor, one has to preliminarily kill one’s own bone marrow, which is undoubtedly a harmful procedure that can be avoided if the bone marrow donor and the recipient are the same person.
What could be expected from heterochronous autologous hematopoietic stem cell transplantation? Based on available datait is assumed that young autologous stem cells that remain cryopreserved for a long period of time will effectively be absorbed into the bone marrow of the same person, while retaining the ability to proliferate and differentiate. Evidence of this is research that has shown that with the introduction of young HSCs to elderly individuals, most of the transplanted cells steadily contribute to blood formation in the long term.
On the part of the innate immune system, it is expected that transplanted HSCs with their healthy “cell progeny” will act favorably by joining the old cells of the innate immune system. With regard to acquired immunity, here transplanted HSCs and the cells produced by them can have many positive effects, for example, by helping to create new cells that synthesize antibodies. Positive effects can be both quantitative and qualitative, which can lead to increased resistance to infections and other environmental and internal pathological problems of the immune system.
Regarding the general pool of stem cells, transplanted HSCs can supplement the quantity and improve the quality of old HSCs, increase their regenerative capacity and increase self-renewal. HSC transplantation can prevent or reduce age-related clonal hematopoiesis and some other age-related abnormalities associated with both the direct function of HSC and the work of other organs, such as the liver and heart.
conducted researchon animals showed the promise of this method. So, in 2013, work was carried out on transplanting old mice (21.5 months) of bone marrow cells from young donors (1.5 months) in an amount of 150 × 10 ^ 6, which accounted for 25% of the total number of mouse bone marrow cells. As a result, the average survival time, starting from the age of 21.5 months, the beginning of the experiment, was +3.6 and + 5.0 (± 0.1) months for the control and experimental groups, respectively, which corresponded to a 39 ± 4% increase in the lifetime experimental group over control.
In recent years, positive results have been obtained.use of HSC transplantation in the treatment of severe autoimmune disease - multiple sclerosis. Patients with an aggressive form of multiple sclerosis, along with chemotherapy, underwent HSC transplantation to restore immune function. According to a multicenter observational retrospective cohort study in 2017, which was attended by 281 patients from 25 clinics in 13 countries, HSC transplantation prevented further development of multiple sclerosis and contributed to the survival of patients, which shows the effectiveness of HSC transplantation to restore immune function.
Although, it is quite obvious that for the application of HSC in the fight against aging, additional research is still needed, which will show the effectiveness and ensure the complete safety of this method.
In general, all the data available today shows quite encouraging effects of stem cell replacement therapy on mitigating and preventing severe pathologies strongly associated with mortality (cardiovascular and neurodegenerative), and on restoring the immune system function. Also, stem cells showed their effective properties in the fight against fibrosis, which further strengthens our hope for cell therapy in the fight against aging in general and age-related pathologies in particular. And in our opinion, combining cell therapy with senolithic or senomorphic (in short, we talked about them) can show an additional good result, removing senescent cells from tissues and regenerating stem tissues.
Mikhail Batin, Timofey Glinin, Alexey Rzheshevsky.

Senolithic - killer cells of decrepit cells
In one of the previous reviews, we talked in detail about the senescent cells and how scientists discovered these cells for the weak. But in short, these cells appear after damage, in the event that the cell could not heal the damage, and at the same time could not start the process of apoptosis and destroy itself.
A decrepit cell cannot divide, performs its functions poorly, and the most unpleasant thing is that it secretes signaling substances that help the surrounding cells to become decrepit. Today, the best approach to blocking this mechanism of aging is considered to be the killing of senescent cells, because even in the old organism there are few of them, and no important functions will be lost. For the removal of such cells, special preparations were developed, called "senolitics" (from senile - decrepit and lytic - destructive).
This research began in 2011. Then James Kirkland and his colleagues conducted an experiment surprising in its complexity and elegance. They have developed a line of specially modified transgenic mice INK-ATTAC (INK-linked apoptosis through targeted activation of Caspase).
The cells of these rodents were subject to apoptosis, cell suicide, associated with p16 Ink4a protein and targeted activation of caspase. Protein p16 Ink4a shows its activity in decrepit cells, blocking the ability of cells to divide. And because apoptosis had a targeted "senoliticheskuyu" action, without affecting normal cells. Activation of caspase initiating apoptosis occurred after administration of the special drug AP20187 to mice.
The results of this workshowed that removing senescent cells from the body delayed the development of age-related pathologies: “ In adipose tissue, skeletal muscles and eye cells, in which p16 Ink4a contributes to the acquisition of age-related pathologies, the removal of p16 (Ink4a) -expressing cells delayed the onset of pathological processes. In addition, the delay at the end of life weakened the progression of already established age-related disorders. These data indicate that cellular aging is causally related to the occurrence of age-related pathologies, and that removing aging cells can prevent or slow down tissue dysfunction and prolong health. »

James Kirkland MD, PhD
After that, James Kirkland became a real leader in the study of Senolithic and in fact monopolized this area of research for the next five years, constantly publishing in the best journals of the world all new molecules with senolytic activity.
In 2014, scientists described one of the mechanisms that regulates cellular aging and can be used in the therapy of senescent. The focus of their work was the DBC1 protein (Deleted in Breast Cancer 1), which regulates several nuclear proteins, including the well-known gene regulator of aging SIRT1. It changes activity in various tissues: with a decrease in DBC1 expression, SIRT1 activity increases and vice versa. It is known that a diet with a high fat content enhances the expression of DBC1, reducing the expression of SIRT1 in the liver and leading to its defeat, steatohepatitis.
In addition, it is known that SIRT1 proteins can suppress cellular aging. Preadipocytes of mice with knockout of the DBC1 gene after 12 weeks of feeding with a high fat content showed fewer signs of cellular aging . They had lower levels of p16 Ink4a involved in stopping the cycle, as well as markers of the secretory phenotype associated with aging (SASP): MCP-1, TNF-α and IL-6. These substances are the very same set of harmful molecules that cause all nearby cells to age.
In addition, a smaller amount of γ-H2AX, a known marker of DNA damage, was detected in preadipocytes.

The mechanism of interaction between the DBC1 and SIRT1 genes, and that is what therapy is aimed for.
In 2017, an article was published in the best scientific journal ScienceHarvard scientists describing a method for suppressing DBC1 by administering nicotinamide mononucleotide (NMN) to mice. In addition, it was found that the suppression of DBC1 also has a beneficial effect on the activation of the DNA repair system after injury. By the way, in its natural form, the NMN enzyme, which was injected into mice, is contained in broccoli.
It is important to understand that in some cases the transfer of a cell to a state of decrepitude, which makes DBC1 protein, can prevent the cell from degenerating into a tumor. Therefore, scientists continued to focus the main focus of research on the suppression of the p16 protein (Ink4a).
It was for killing cells with this marker that the first senolitic drug was discovered. It was a combination of two substances: dasatinib, an anticancer agent with directional action, and quercetin - a flavonoid with antioxidant and anti-inflammatory properties.
In the course of the study, scientists tested 46 potential drugs aimed at activating cellular suicide, apoptosis, in aging cells. As a result, dasatinib and quercetin showed the best result.
Quercitin and Dasatinib
First, a combination of these drugs (dasatinib 5 mg / kg and quercetin 50 mg / kg) was given orally to older mice (24 months old).
After 5 days, preadipocytes and endothelial cells showed a decrease in the levels of cell aging markers - a special form of beta-galactosidase (SA-βgal) and mRNA of the p16 gene. In addition, there was an improvement in cardiovascular and physical function in older mice.
The scientists then tested the effects of dasatinib and quercetin on a mouse model of accelerated aging. Introduction of senolithic to such mice resulted in a decrease in the expression of aging markers in several tissues, a decrease and weakening of the signs of aging were observed in general: spinal curvature decreased, tremor, urinary incontinence, gait disturbance, hind limb paralysis, etc. The healthy period of life increased.
Dasatinib and quercetin also removed senescent cells from irradiated tissues.

All the data obtained allowed to state that “senolithics can be used in the future to prevent cardiovascular diseases, senile asthenia, as well as slow recovery or dysfunction after chemotherapy or radiation, neurodegenerative disorders, osteoporosis, osteoarthritis, other diseases of bones and joints and adverse pathologies related to chronological aging »
Follow-up researchconfirmed and expanded the data obtained on the senolytic properties of dasatinib and quercetin. Rodent treatment with dasatinib and quercetin improved the ejection fraction of the heart and increased vascular reactivity in old mice after one 3-day course of treatment. Also, their introduction reduced vascular calcification and improved the condition of the vessels with a high-fat, hypercholesterolemic diet.
Dasatinib and quercetin improved pulmonary function and decreased pulmonary fibrosis in a mouse model of idiopathic pulmonary fibrosis, reduced liver steatosis caused by a high-fat diet, and reduced osteoporosis in old mice.
In a mouse model of human progeroid syndrome, dasatinib and quercetin reduced brittleness, osteoporosis, loss of glycosaminoglycans in the intervertebral discs, and spondylosis.
Recently, in January of this year, the results of the first open pilot study of senoliths in humans were published . The combination of dasatinib and quercetin improved physical performance in elderly people with idiopathic pulmonary fibrosis.
Navitoclax
In 2016, Kirkland and colleagues presented another potential senolithic drug, navitoclax (ABT263). Navitolax is an inhibitor of proteins of the Bcl-2 family, which are involved in the regulation of apoptosis, and is undergoing clinical trials as an antitumor drug. It revealedthat navitolax, inhibiting the activity of Bcl proteins, stimulated apoptosis in some old cells. It acted as a senolithic on human umbilical vein endothelial cells (HUVEC), on human lung fibroblasts (IMR90) and mouse embryonic fibroblasts (MEF), but had no senolytic effect on human preadipocytes.
In the same year, another group of scientists described the effect of navitolax in experiments with rodents that normally aged and were exposed to radiation. Oral administration of navitolax to irradiated and aging mice effectively removed senescent cells, including aging bone marrow hematopoietic stem cells and muscle stem cells. The authors made this conclusion from their study: “Our results show that the selective purification of senescent cells using a pharmacological agent is partly due to the rejuvenation of old stem cells of various tissues. Thus, senolithic drugs can be a new class of anti-aging and anti-radiation agents . ”
In 2017, the ability of navitolax to influence the development of fibrosis was demonstrated . Navitolax removed apoptosis-resistant myofibroblasts that participated in the formation of fibrosis in a mouse model of scleroderma (an autoimmune disease characterized by multiorgan fibrosis).
Tocotrienols
For the last three years, the senolitic effect of tocotrienols, chemicals from the vitamin E family has been described in detail. For a long time, tocotrienols were in the shadow of other chemicals of the same family, tocopherols, and their active study began quite recently. As it turned out, tocotrienols have great potential in the fight against various diseases and aging. They have a pronounced antioxidant and neuroprotective activity.
Their ability to exert a protective effect on neurons and alleviate the symptoms of Parkinson’s disease has been shown. Also tocotrienolscan help prevent bone loss due to osteoporosis and the onset of stomach ulcers and gastritis due to stress. In addition, tocotrienols had a prophylactic effect on the development of cardiovascular pathologies and had antitumor properties, stimulating apoptosis in cancer cells.
This last property, induction of apoptosis, allowed scientists to view tocotrienols as potential senolitics. Why it was conducted a number of studies , showing them senoliticheskuyu efficiency . Based on all of the above, it does not seem at all accidental that in the title of one of the scientific articles, tocotrienols are designated as “vitamin of the 21st century” with largeclinical capabilities .
Perhaps it would not be a great exaggeration to say that tocotrienols possess one of the most significant potentials as senolytic agents.
Another class of senolithic, found by James Kirkland and his colleagues, were inhibitors of the protein Hsp90 (Heat shock protein 90). Hsp90 belongs to the heat shock protein family. They perform the function of chaperones in the body (from the French shaperon - nanny), participating in folding (folding into the correct structure), degradation and stabilization of the protein, correcting errors in the protein structure.
But, as is often the case, Hsp90 has a dual role in the body: useful and not very. In addition to protecting vital proteins, Hsp90 supports proteins involved in carcinogenesis - it stabilizes several unstable oncogenic factors, such as mutant EGFR, BRAF and HER2, as well as some anti-apoptotic factors, preventing the removal of "damaged" cells.
Kickrland and colleagues found that inhibitors (i.e., activity suppressors) of the Hsp90 protein (geldanamycin, tanesimycin, 17-DMAG, etc.) also have senolytic properties. The mechanism by which Hsp90 inhibitors removed old cells included the effect on the activated form of AKT protein kinase, which suppresses apoptosis, affecting MTOR, NF-kB, Foxo3a, and other signaling pathways in cancer and old cells.
The introduction of transgenic mice with accelerated aging of one of the Hsp90 inhibitors, 17-DMAG, mitigated and eliminated the occurrence of several inherent age-related symptoms: kyphosis, dystonia, tremor, poor coat, ataxia, gait disorder, etc. According to the authors of the study, their results show that the HSP90 protein is a real molecular target for the destruction of senescent cells .
Finally, one of the latest discoveries on the “senolithic front” is the senolithic fizetin, presented in September this year by the same Kirkland and his team. Physetin is a famous flavonoid contained in many vegetables, fruits and berries (cucumbers, strawberries, persimmons, grapes, onions, etc.).
The objective of this study, according to the authors, was to identify another flavonoid with stronger senolitic properties than quercetin. A total of 10 flavonoids were analyzed. In senescent mouse embryonic fibroblasts and in human fibroblasts, fisetin most effectively lowered the levels of cell aging markers. Then he was examined on animals.
In the progeroid mouse model, administration of fizetin significantly reduced the expression of cell aging markers in all tissues. A decrease in the expression of p16 Ink4a, p21 Cip1 and SASP factors in peripheral blood CD3 + T cells, a cell type that demonstrates a steady increase in the expression of p16 INK4a in old people, was also observed. In addition, fizetin reduced oxidative stress in the liver, acting as an antioxidant and increasing levels of another antioxidant, intracellular glutathione.
In normally aging old mice, fizetin also reduced markers of cellular aging, but, most importantly, caused an increase in the life expectancy of rodents. The authors of the work describe their discovery with great enthusiasm: “Our results show that physetin is aimed at multiple, but not all types of aging cells in the body. In addition, by reducing the percentage of aging cells, Fizetin reduces the expression of aging markers in several organs. This leads to an improvement in tissue homeostasis and a reduction in multiple age-related pathologies, which is consistent with the impact on the fundamental aging process.
The fact that fizetin has reduced the proportion of aging T and NK cells can help strengthen the positive effects of physietin, since healthy immune cells are important for cleaning aging cells. Likewise, Physuetine reduces markers of inflammation and oxidative stress. ” Considering that physetin is a natural product found in food products and no adverse side effects have been found for it, the authors of the study suggest that physetin can soon be used in the treatment of humans .
And most recently , in November of this year, British scientists presented two more potential senolitik: antibiotics roxithromycin and azithromycin, which are aimed at aging human fibroblasts.
In the light of the foregoing, it seems quite logical that the elimination by senolithic cells of senescent cells in the elderly should improve health and increase longevity. Experiments with rodents modified INK-ATTAC (which we mentioned at the very beginning) showed that cleaning the body of a part of old cells by administering ap2014-activating drug AP20187 increased the life of mice compared to the control group by 17–35%. Decrease in markers of cellular aging was recorded immediately in several tissues: in the lungs, heart, kidneys, skeletal muscles, spleen. In addition, the function of the heart, kidneys and eyes remained normal in rodents , good physical activity and slowed tumor genesis.
Stem cells
In our opinion, after the treatment with senoliths, it seems quite logical to carry out the second stage, which helps to restore organs and tissues, to carry out replacement cell therapy with stem cells.
Stem cells used in cell therapy come in several forms. The most promising use of cell therapy may be in the treatment of age-related bone diseases, in pathologies that are poorly amenable to medical treatment (cardiovascular and neurodegenerative), as well as in the restoration of immune function. Consider briefly these three areas of cell therapy.
Mesenchymal stem cells (MSCs) are believed to have the greatest potential for tissue repair in cardiovascular diseases. MSCs were opened half a century ago by Soviet scientists, Alexander Friedenstein and his colleagues.
They have their own characteristic features: the ability to symmetric and asymmetric division, high proliferative potential and ability to adhere, fibroblast-like morphology, the formation of colonies in culture and easily induced differentiation. It is their last property, discovered not so long ago - the ability to differentiate into different types of cells - makes MSCs the most appropriate tool in cell therapy.
Another little-used type of stem cells is hematopoietic stem cells (HSC), which are involved in the formation of the immune system and blood formation and are only found in the bone marrow. Despite the fact that they are constantly divided in the bone marrow, there are relatively few of them, and they are rarely considered as an instrument for the treatment of aging. Their typical field of application is for bone marrow transplantation after surgery.
In the bone marrow, undifferentiated MSCs create a microenvironment for another type of bone marrow stem cells - hematopoietic stem cells, producing matrix molecules: fibronectin, laminin, collagen, etc. Also, MSCs secrete cytokines necessary for differentiation of HSCs. In cell therapy,
MSCs are used in three main areas:
- Hematopoietic support in joint transplantation with HSC;
- Replacement and restoration of function of damaged non-hemopoietic tissues (bones, cartilage, skeletal muscles, cardiac muscle, nervous tissue, liver, etc.);
- Suppression of immune conflicts with allogeneic unrelated transplantation and severe autoimmune processes.
Due to its great plasticity, MSCs can differentiate into a wide range of cells of various types: into striated and smooth muscle cells, into cardiomyocytes, into cells of bone and nervous tissue, liver, kidneys, pancreas, immune system cells and many others. In fact, we are talking about the whole reserve stem cell hospital in our bone marrow, ready to help in a wide variety of emergency situations. In experiments with animalsit was traced how the labeled MSCs introduced into the body were then detected in the cells of the brain, heart and other organs. A characteristic feature of MSCs is their predominant introduction into tissues with foci of damage, which demonstrates their regenerative potential. This is also true for the treatment of heart diseases, the cells of which, after injuries, do not have the ability to regenerate themselves.
If you take as an example of heart disease, then there are several criteria why MSCs are suitable for the treatment of heart attacks :
- The ability of cells to differentiate into cardiomyocytes, contain contractile structures;
- The presence of interstitial insertion disks between cells for conducting excitation potentials to the transplanted cells from the host cardiomyocytes;
- No rejection reaction;
- Minimal ischemic damage (shock, necrosis, and apoptosis of the transplanted cells);
- The ability of transplanted cells to repair the damaged area (replacement of connective tissue and the formation of a complete biomechanical architectonics of cardiac tissue).
There are three main mechanisms of action of MSCs that underlie their beneficial effects on the heart:
- Reduction of fibrosis;
- Stimulation of angiogenesis;
- Restoration of contractile function through engraftment and stimulation of the endogenous stem cells of the heart.
Thus, already conducted clinical studies have shown that the introduction of MSCs to patients after myocardial infarction improved heart function (left ventricular function), improved physical condition and reduced mortality [22-24].
It is known that cardiac pathologies are accompanied by the death of cardiomyocytes. The introduction of MSCs is accompanied by a decrease in the apoptotic processes in the heart and scar reduction. Also, the work performed showed that MSCs play a large role in the processes of antiogenesis (the formation of new blood vessels), which are extremely important in the restoration of cardiac tissue.
In addition, MSC transplantation stimulated the proliferation and differentiation of endogenous heart stem cells, which are involved in the replacement of damaged tissue with a new one, capable of normal contraction. Over the past 15 years , a number of preclinical (in animal models) and clinical (in humans) have been carried out , showing the effectiveness of MSC in heart disease.
Another promising direction in the use of MSCs is the therapy of neurodegenerative pathologies, the medical treatment of most of which does not exist today. It has been established that MSCs can cross the blood-brain barrier and migrate to different areas of the brain.
The experimental studies described the participation of MSCs in the regeneration of damaged brain structures. Under the influence of microenvironment factors, introduced MSCs integrate into the damage area, form numerous contacts with neurons, acquire a neuronal phenotype (expression of nestin, a specific enolase, tyrosine hydroxylase, etc.) and functional activity of dopaminergic neurons. MSCs also have a neuroprotective effect, differentiating into microglial cells that provide trophic support for damaged tissues. MSCs activate the processes of neurogenesis by stimulating the neuronal stem cells of the subventricular zone of the brain, which then migrate, proliferate, differentiate and replace dead dopaminergic neurons.
According to scientists, today we can distinguish 3 main results of studies of cellular therapy for neurodegeneration:
- Stem cell therapy is probably the only possible treatment that offers “treatment” for neurodegenerative diseases.
- Structural and functional improvements observed in animals require additional research. The long-term clinical outcome and safety of the treatment of neurodegenerative diseases of stem cells requires further research (some are undergoing right now).
- Of the 4 types of the most common neurodegenerative diseases, there is relatively more evidence for stem cell therapy in Parkinson's disease and amyotrophic lateral sclerosis compared with Huntington and Alzheimer's disease.
Over the past 10 years, a number of preclinical studies in animal models have been carried out, showing a beneficial effect of cell therapy on neuropathology. Thus, a positive therapeutic effect was demonstrated from the intravenous administration of human MSCs isolated from adipose tissue to transgenic mice with Alzheimer's disease.
To check the migration of MSCs introduced into the brain, the researchers labeled them with fluorescent magnetic nanoparticles. Fluorescence signals from organs extracted 3 days after the transplantation of cells showed that the cells had spread to all organs, including the brain. After dissection of the brain into 5 separate areas (olfactory bulb, hippocampus, cerebellum, brain, midbrain and cortex), fluorescent signals of nanoparticles were detected in all areas of the brain, except for the olfactory bulb.
The positive effect was manifested in the improvement of cognitive abilities (learning and memory), the acceleration of endogenous neurogenesis and the increase in synaptic stability in the rodent brain. It was also recordedreducing the number of amyloid plaques in the brain, reducing the levels of beta-amyloid and the terminal fragment of amyloid precursor and reducing the apoptotic processes in the brain.
In another study, scientists injected MSCs from adipose tissue in mice with simulated amyotrophic lateral sclerosis. The outcome of the therapy was a slowdown in the development of movement disorders, an increase in the number of lumbar motoneurons and neuron growth factors.
StudyEffects of cell therapy on the treatment of Parkinson's disease was performed on rodents and monkeys. In rats with rotenone-induced Parkinson's disease, stem cell therapy caused immunomodulatory, anti-inflammatory and neurotrophic effects. Rodent serum levels of transforming growth factor β, monocyte chemoattractant protein and levels of dopamine in the brain increased. Therapy of MSCs from adipose tissue in combination with adenovirus containing NTN and tyrosine hydroxylase (TH) (Ad-NTN-TH), rhesus macaques with the Parkinson's disease model led to neuroprotective effects, reduced tremor and increased mobility.
These results are particularly important, since fatty tissue in significant quantities (up to 300 ml or more) can be obtained under local anesthesia with relatively little painful cosmetic liposuction, lipoaspiration of subcutaneous fat, or by excision of fat deposits. This tissue serves as a source of MSC for transplantation and tissue engineering.
In addition to the cardiovascular and neurodegenerative pathologies discussed above, cell therapy has great potential in restoring immune function, which, as is well known, worsens with age. For these purposes, another type of bone marrow stem cells is used - hematopoietic stem cells (HSC).
The immune system is closely intertwined with the nervous and endocrine systems, carrying out the regulation of all body processes, including making a huge contribution to the aging process. In addition, the deterioration of the functions of immunity with age makes a person poorly protected by various kinds of infections, worse purifies the body from old cells and can lead to the development of autoimmune and inflammatory processes. All this, naturally, shortens the life span and accelerates aging.
It is logical to assume that maintaining immunity is normal is one of the main strategies for combating aging. One of the mechanisms that can keep the immune system in good condition is bone marrow cell transplantation. The concept of this method is based on enhancing the aging immune system by young, healthy, autologous (ie, own) hematopoietic stem cells (HSC), which were collected from the same person in his youth and cryoconserved for a long period. Upon reaching old age, these own stem cells introduced into the body can rejuvenate the immune system and improve immune functions. This process is called autologous hematopoietic stem cell transplantation (haHSCT).
HSC transplantation itself has a half-century history and has been successfully used to restore immune function in the treatment of cancer and autoimmune diseases. About 40 thousand HSC transplantations are done every year in the world, the total number of which has reached 1 million over the entire period of the study. Studies have shown that HSC transplanted are included in the hematopoietic and immune functions of the recipient that were irradiated or chemically immunized during chemotherapy. The transplanted HSCs colonize the bone marrow, thereby establishing the complete set of cells necessary for the normal immune and hematological status of the patient.
At the same time, when a HSC is transplanted from an outside donor, one has to preliminarily kill one’s own bone marrow, which is undoubtedly a harmful procedure that can be avoided if the bone marrow donor and the recipient are the same person.
What could be expected from heterochronous autologous hematopoietic stem cell transplantation? Based on available datait is assumed that young autologous stem cells that remain cryopreserved for a long period of time will effectively be absorbed into the bone marrow of the same person, while retaining the ability to proliferate and differentiate. Evidence of this is research that has shown that with the introduction of young HSCs to elderly individuals, most of the transplanted cells steadily contribute to blood formation in the long term.
On the part of the innate immune system, it is expected that transplanted HSCs with their healthy “cell progeny” will act favorably by joining the old cells of the innate immune system. With regard to acquired immunity, here transplanted HSCs and the cells produced by them can have many positive effects, for example, by helping to create new cells that synthesize antibodies. Positive effects can be both quantitative and qualitative, which can lead to increased resistance to infections and other environmental and internal pathological problems of the immune system.
Regarding the general pool of stem cells, transplanted HSCs can supplement the quantity and improve the quality of old HSCs, increase their regenerative capacity and increase self-renewal. HSC transplantation can prevent or reduce age-related clonal hematopoiesis and some other age-related abnormalities associated with both the direct function of HSC and the work of other organs, such as the liver and heart.
conducted researchon animals showed the promise of this method. So, in 2013, work was carried out on transplanting old mice (21.5 months) of bone marrow cells from young donors (1.5 months) in an amount of 150 × 10 ^ 6, which accounted for 25% of the total number of mouse bone marrow cells. As a result, the average survival time, starting from the age of 21.5 months, the beginning of the experiment, was +3.6 and + 5.0 (± 0.1) months for the control and experimental groups, respectively, which corresponded to a 39 ± 4% increase in the lifetime experimental group over control.
In recent years, positive results have been obtained.use of HSC transplantation in the treatment of severe autoimmune disease - multiple sclerosis. Patients with an aggressive form of multiple sclerosis, along with chemotherapy, underwent HSC transplantation to restore immune function. According to a multicenter observational retrospective cohort study in 2017, which was attended by 281 patients from 25 clinics in 13 countries, HSC transplantation prevented further development of multiple sclerosis and contributed to the survival of patients, which shows the effectiveness of HSC transplantation to restore immune function.
Although, it is quite obvious that for the application of HSC in the fight against aging, additional research is still needed, which will show the effectiveness and ensure the complete safety of this method.
In general, all the data available today shows quite encouraging effects of stem cell replacement therapy on mitigating and preventing severe pathologies strongly associated with mortality (cardiovascular and neurodegenerative), and on restoring the immune system function. Also, stem cells showed their effective properties in the fight against fibrosis, which further strengthens our hope for cell therapy in the fight against aging in general and age-related pathologies in particular. And in our opinion, combining cell therapy with senolithic or senomorphic (in short, we talked about them) can show an additional good result, removing senescent cells from tissues and regenerating stem tissues.
Mikhail Batin, Timofey Glinin, Alexey Rzheshevsky.
