Notes phytochemist. Potatoes. Part Three Bulba Fugu or SOLANIN
- Tutorial
... a potato is quite capable of coping with a person.
unknown folk source
The most common "potato threat" is considered solanine, which "forms in green potato" and the next phytomeat is devoted to him, the consequences of his use, as well as methods of salvation from it. Since the information on the Internet is very fragmented, and even it is simply not there - I tried to write in such a way that I got an exhaustive tutorial(it may even get to Wikipedia someday, although Habr would rather become Wikipedia). Therefore, I advise anyone to go under the cut faster than anyone who cares for their health. At the same time, you will find out why the Islamic fundamentalists' manuals teach you to use potatoes as chemical weapons of mass destruction and why pregnant women need to be very careful when using “green potatoes” or even abandon this option to avoid the occurrence of neurological abnormalities in the fetus. UPD : and pro without the danger of sprouted potatoes.

Recently, along with such stable expressions as “antioxidants” or “anti-nutrients”, the concept of phytoalexins is used in world phytochemistry. In this way, researchers are trying to reduce all herbal components that have antimicrobial and often antioxidant properties of substances synthesized de novo plants, which quickly accumulate in the areas of infection with pathogens and are a plant "immune response." As a rule, these substances are inhibitors of a wide spectrum of action with a very diverse chemical structure and specific features inherent in certain plant species. In general, phytoalexins are divided into several classes: terpenoids, glycosteroids, alkaloids. Many researchers are in favor of expanding the definition of "phytoalexin" and the inclusion in this concept of all phytochemical components,
In English, the Solanaceae family (the same family, which includes our beloved potatoes, tomatoes, eggplants, sweet peppers, but also tobacco, mandragora, belladonna, henbane and dope) is also called nightshades, literally "night shadows." The appearance of this name is sometimes attributed to the fact that even the ancient Romans actively used the plants of this family to prepare various poisons. This makes sense, since in my opinion, the most powerful weapon of plants is their alkaloids. In essence, alkaloids are common chemical nitrogenous compounds, most often in plants acting as a secondary metabolite. In plants of the Solanaceae family, the most bioactive are the so-called. tropane alkaloids (scopolamine, atropine, hyoscyamine, nicotine) and precisely because of their bioactivity, these compounds have been used most often since ancient times as poisons (belladonna or henbane poison, the psychoactive effect of dope, mandrake and tobacco).
Note : it is scopolamine, the alkaloid contained in such plants as scopoly, belladonna, henbane, dope, at the beginning of the 20th century was proposed as a “truth serum”, a disinhibiting effect was caused by the introduction of so-called state of "twilight consciousness". This, of course, is not quite the effect, but nevertheless ...
Solanine is a class of alkaloids of plants of the Solanaceae family, which is mostly present in potatoes.
Note : it is often possible to find a statement that solanine is also found in tomatoes. This is not true. Tomatoes contain “something similar” - an alkaloid tomatin (or lycopersicin). It is tomato and its derivative, tomato, that play a major role in confronting a tomato in front of a fungal, microbial, insecticidal (because of the presence of tomato, the tomato does not take on the Colorado potato beetle) (well, of course, from snails and goats ). Therefore, most of the alkaloid is concentrated in the leaves and inflorescences, approximately 1/5 falls into the fruit (and when ripe it gradually decreases to a certain indicator depending on the variety). Why I decided to mention tomato. Firstly, it is almost always confused with solanine, although tomato is ten times less toxic than solanine (LD 50oral intake of about 500 mg / kg body weight). And secondly, it illustrates what every phytosterol aspires to "- when in contact with cholesterol in any product, it instantly binds it into an insoluble complex and removes it from the body without digestion. In general, if the hands reach the phyto-consideration of tomatoes, I will tell you more about this interesting alkaloid.
We return to the solanine. This glycoalkaloid was discovered as early as 1820 in Black Nights ( Solanum nigrum ) berries , after which it was named. It belongs to the chemical family of saponins. I once wrote about saponins in one of my banana notes:
Under natural conditions, the cycle of alkaloids constantly occurs in potatoes, i.e. during the growth of the plant, tens and hundreds of different compounds are biosynthesized. Naturally, there are many alkaloids among them, including the already mentioned solanine. Solanine together with its related compound, haconin, is synthesized in potatoes from acetyl-coenzyme A and cholesterol. The scheme by which all this happens can be seen below (the image is clickable).

The mentioned alkaloids are constantly synthesized as a kind of “depot protection” against insects, fungi, microorganisms and herbivores.
Minute lyrics. Here I look at these biosynthesis schemes and I understand that chemistry should strive for just such a type. Necessary compounds, peptides, etc. - synthesized in plants, not in flasks. And the chemist's laboratory is some kind of experimental field, not a laboratory room in which you can hang an ax. I don’t know how quickly my dreams will come true, but my favorite D. Cronenberg, in his Existency ( eXistenZ ), was already growing a weapon inside a living reactor ...
OK, continue. The greatest accumulation of protective alkaloids is observed in those parts of the plant that are most at risk of being eaten (ie, in parts located above the ground) - in the leaves, stems, shoots and inflorescences. Under normal (= "greenhouse") conditions, only a small amount (relative to the amount in the remaining parts) of the alkaloids is synthesized in tubers.
But the situation changes dramatically when the tuber is pulled out of the ground, they begin to be transported and stored in anthropogenic conditions. At the beginning of the article I wrote about phytoalexins, as substances that start to generate plants when they get into unfavorable conditions (uncomfortable) environmental conditions or are exposed to damage (mechanical, fungal, bacterial, etc.). The response of a plant to such factors is reflected in an increase in the production of glycoalkaloids (sometimes several times). Actually, the entire mechanism of biosynthesis works only for “war”.
The most common "folk" sign of the presence of high solanine content is the green color of the potato.

IMPORTANT : although the green color of the potato, which was exposed to light radiation, is only the color of chlorophyll (which, by the way, is actively used as a dye in food products, like cheese , and completely harmless). Solanine, which is synthesized in such tubers (after all, a bright light = “tuber on the surface” => include protection against eating = “synthesize killer doses of solanine and hakonin) in general does not have color and it is impossible to notice it with the naked eye. As in the case of a banana - this is just a color indication of a possible (but not mandatory) increase in the level of glycoalkaloids. Those. Each of the processes (accumulation of solanine and greening of potatoes) can quite do without the other.
By the way, Ukrainian researchers experienced this inconsistency earlier than others and began to create a biosensor on for the rapid detection of glycoalkaloids, which exploits the anticholinesterase activity of glycoalkaloids. True, despite the fact that more than 10 years have passed, "things are still there." So - we work in the old manner.
Continuing the theme of greening, we can safely say that “and my grandmother was right again,” when she advised me to cut the peel of green potatoes into white flesh. Indeed, in potato tubers from 30 to 80% of the solanine / hakonin fraction content falls on the peel and the layer of pulp under it is about 7-10 mm (i.e., on the skin, bark and vascular ring). To better understand what to trim - below is a graphic illustration.

In addition to sunlight, fungal diseases (all kinds of “rot”, etc.), as well as damage to tubers during harvesting and transportation, also play the role of aggressive external factors that lead to “corned beef”. By the way, a small life hack. Saponins are generally known for having a very bitter taste, which frightens herbivores even in small doses. This is completely true for solanine. Those. if a bitter taste is observed in a green potato, such a vegetable is only for ejection (it is absolutely impossible to feed even cattle). It is bitterness that is considered the most reliable criterion for the presence of solanine (although I would partially argue, since some short-chain peptides formed during the incomplete enzymatic hydrolysis of potato proteins and which can be foundin raw potatoes, they also give a bitter taste, but these are chemical particulars, in general - bitter potatoes will definitely be harmful to the body).
UPD : the Solairw user , in his commentary, touched upon the fact that I initially missed from attention, about “potatoes with sprouts”, i.e. sprouted potatoes, "about the danger of which is also often said."

When storing potatoes, the process is so-called. aging, during which some biochemical processes in the tuber take place slowly but surely. If the external conditions (temperature, humidity, light) are within the physiological "potato" ratethen there is no noticeable deterioration in the quality of the tuber. Yes, there is a gradual decrease in starch and total sugar, yes, there is a proteolysis of patatin potato protein. But, for example, the content of antioxidant components practically does not change, and all protein compounds are inactivated during heat treatment (during cooking / frying). A completely different situation arises if, due to improper storage conditions, the tubers are exposed to cold / heat shock (stress) or excessive light exposure. As a response to such factors unfavorable to the tuber, the defense mechanism is activated and the potatoes begin to grow green and germinate. As a rule, this is accompanied by enhanced biosynthesis of solanine / haconin glycoalkaloids, and they are localized mainly in those same shoots (read “shoots take a hit”). What does this mean for the average consumer? And all the same. In principle, everything said in the article is equally applicable for sprouted potatoes and for non-sprouted. It is just worth removing the sprouts together with the greened skin, thus minimizing possible contact with poisonous alkaloids and then just boil / fry the potatoes as if nothing had happened. Of course, such potatoes have slightly deteriorated taste (less starch / sugars), but then the glycemic index will be lower. Most people consider sprouted potatoes to be harmful and immediately discarded, but I note that it is much less harmful than not sprouted, but damaged by various kinds of rot. The fact that the problem really exists is confirmed by the attention paid to such a respected organization, as the US Department of Agriculture (the nutrient base of which I constantly use when writing my articles). Every year, hundreds of tons of sprouted potatoes are thrown into the United States, which is still quite possible to be eaten without much harm by simply processing it correctly. In the form of confirmation under a spoiler a piece of the methodical "potato" recommendations of the USDA.
Due to the high prevalence of potatoes (and therefore the availability of skills to work with them among consumers), cases of solanine poisoning are quite rare under normal conditions (i.e., when there is enough food). The danger manifests itself in areas of humanitarian disasters, armed conflicts, etc. where food shortages. But even in such cases, the removal of 10–15 mm of peel from each tuber will relieve the “gourmet” from a large dose of glycoalkaloids (the rest will be removed by heat treatment, but more on this below).
About what solanin arises from and why he does it, I told. It would be logical to assume that the reason for its deadly harm should be explained. This will be true for both saponins and other surfactants (surfactants). In their action, glycoalkaloids put pressure on two diseased sites - they destroy cell membranes and inhibit the enzyme acetylcholinesterase (even in rats they induce destruction of the liver, but “this is not accurate”).
The mechanism of action on the cell membrane is shown in the picture (the image is clickable)

By their poisonous effect on the cell membrane, glycoalkaloids are required to form stable stoichiometric (1: 1) complexes with cholesterol, which in turn is one of the most important cellular substances (and not just “cholesterol plaques”, as someone might think). After entering the body, the alkaloid part (aglycone) of solanine reversibly binds to sterols, which form a double lipid layer (read about this layer in the last banana note , and a reminder picture under the spoiler)
When the density of glycoalkaloids in the membrane reaches a certain level, the “sugar” carbohydrate heads of our “potato poisons” will begin to electrostatically attract each other and form already stable and completely irreversible glycoalkaloid-sterol complexes. In the place of sterols lost to the organism, sterols from the inner side of the double-lipid layer destroyed by alkaloids will be pulled from the inner side (to “mend” the gap that has formed) ”. As a result, roughly foam bubbles form in the double lipid layer, its integrity and performance are impaired. The cell in which the membrane is destroyed - perishes. For example, when glycoalkaloids enter the intestine and disruption of the double lipid layer of the membrane of epithelial cells, macromolecules of everything else (including the alkaloids themselves) begin to flow into the blood. Actually, glycoalkaloids destroy one of the main cellular protective barriers of the body. In addition, the damaged membrane changes its potential and reduces the active transport of sodium ions, which affects, for example, cardiac activity. Solanine is able to "open" potassium channels of mitochondria, increasing their membrane potential. This, in turn, leads to the transportation of Ca2+ from mitochondria into the cytoplasm, and such an increase in the concentration of calcium ions in the cytoplasm causes cell damage and apoptosis .
The second poisoning effect is the inhibitory effect on the enzyme acetylcholinesterase . Here is what Wikipedia says about this :
A rather unpleasant effect is described in the article . The authors established an epidemiological correlation between the congenital malformations of the fetal nervous system (anencephaly and vertebral artery cleft) in pregnant women and the use of potatoes with increased glycoalkaloid content in women.
Main symptomssimple poisoning with solanine are mainly gastrointestinal manifestations, i.e. vomiting, diarrhea, abdominal pain (therefore, there are cases when poisoning with solanine was taken for gastroenteritis) + in acute poisoning, symptoms typical for poisoning with ARS with acetylcholinesterase inhibitors can also be added (as well as fever, rapid pulse, low blood pressure, rapid breathing, etc.). Estimates of LD 50 toxicity vary, it is believed that a dose of 2–5 mg / kg body weight causes toxic manifestations, and 5–10 mg / kg can be fatal. There is even a tacit guide that recommends the concentration of glycoalkaloids = <200 mg / kg wet weight when developing new potato varieties.
Note: it will be wrong to show only the dark side of power. Therefore, I note that some acetylcholinesterase inhibitors are even synthesized artificially for use in the treatment of Alzheimer's disease, dementia with Levi's calves, myasthenia, can be used as an antidote for anticholinergic poisoning (cyclodol, dimedrol, taren). Comparatively recently studies have been conducted.on cell cultures, which showed high efficacy (comparable to the anticancer drug adriamycin) of solanin and hokanin in inhibiting the growth of cancer cells in tumors of the colon and liver in humans. The anti-cancer effect of glycoalkaloids was also observed when using cell cultures of cervical cancer, lymphoma and stomach cancer. Moreover, the use of two or more glycoalkaloids allowed to achieve a stable synergistic effect. There is also a work where it has been shown that the use of haconin reduces the number of metastases in lung cancer and may in the long run create a new direction of chemotherapy.
Who is to blame, I hope, clearly. But what to do? And to ask permission from the potato to eat it, as in the old days, people asked permission from the tree to cut it down in order to build a house. Moreover, it is better to tell the tuber how to spend the power that you will get from the starch. Whether the silushka will go to good business. And having eaten potatoes, you feel, as you feel, the tuber, as it were understood by you, the test “learn your eloquence for plants”. Treat the potato with respect, and he will not poison you with solanine.
But seriously, it is very likely that the attentive reader has already partly understood from the text of the article how to protect themselves from being poisoned by solanin & hokanin. Firstly, you need to choose potatoes with minimal mechanical damage (it’s not for nothing that people in shops intuitively buy clean, well-groomed potatoes from Israel, and don’t take a Belarusian one, which is not only expensive, but also scratched, potholed, rotten) signs of fungal and bacterial diseases. Secondly, if there are suspicions of the presence of harmful glycoalkaloids - just remove the peel to a depth of 1-1.5 cm (of course, nothing can remain in the end from small potatoes), this will remove up to 80% of the potato alkaloids.
Important!: The popular methods of heat treatment (cooking, frying, baking), on the contrary, have a weak and “with varying success” effect on the content of solanine and its comrades. For example, cooking potatoes reduces the level of α-haconin and α-solanine by only 3.5% and 1.2%, respectively (but at the same time, cooking in a microwave reduces the content by 15%). Deep roasting at 150 ° C does not lead to any tangible changes, significant destruction of glycoalkaloids begins only at ~ 170 ° C, and frying at 210 ° C for 10 minutes results in a loss of 40% of harmful alkaloids (another thing is then there will be such potatoes). Freeze drying or other types of dehydration does not have a significant effect on the content of solanine in general.
Third, the toxicity of alkaloids in food can also be affected by the products in its (food) composition. After all, you can combine them so that solanine and his ilk adsorbed or at least turned into an inactive compound. Glycoalkaloids are generally hard to digest in the body, the presence of fiber (read dietary fiber) interferes with absorption. Folic acid, glucose-6-phosphate and Nicotinamide adenine dinucleotide ( NAD ) bind haconin into non-toxic complexes. The most active form of solanine & haconin is the β-form (see picture above), respectively, all that contribute to the hydrolysis of this type of glycoalkaloids reduces toxicity (and this can be low pH or exposure to mold fungi Aspergillus niger (!!)).
By the way, there is such an interesting direction in food business (to write an article about which Alexey Shukayev long ago asked me ) as food pairing .
It is obvious that a cook with such skills is not a “graduate of a culinary technical school,” but rather a chemist and even a nanotechnologist (as with the Fugu fish, when people have been learning how to prepare it for years). Although I fully admit that there are cooks without a proper background, but with God's spark, who, not knowing any chemistry and biology there, can, on a whim, prepare "ambrosia and nectar" that grant health and cure for any ailments. Namely, this should, in my opinion, be any food (about functional food look in the banana article ).
And fourth. At the very extreme case I will say that in case of acute poisonings with the mentioned alkaloids, cholinesterase reactivators are introduced into the body.. They contribute to the restoration of cholinesterase activity, providing an antidote effect. In the first stage of poisoning, dipyroxime is used . In case of pronounced disorders of mental activity (lethargy, coma), a centrally acting drug, isonitrosin, is additionally administered . At the third stage of poisoning, the combined use of dipyroxime with isonitrosin is necessary.
On this all, take care of yourself and be careful! And to be fully prepared - read Habr (# phytochemist notes) :-)
PS I can not fail to mention the fact voiced in the comments ( one and two times ) by the user minamoto . Let's call it the phenomenon of " potato Lenope". A variety with this name was introduced to the US market in 1967 and already in 1970, these potatoes had to be massively withdrawn from crop rotation. The reason for this was mass poisoning caused by several times too high content of potato glycoalkaloids (16–35 mg / 100 g fresh). masses of potato varieties Lenope versus 3–18 mg / 100 g of fresh mass in potatoes of other varieties.) Interestingly, the variety was bred using traditional breeding methods (this fact is often used as counter-arguments in conversations with opponents of GMO fiction). some torye researchers, exceptionally high levels of glycoalkaloids were due to "impurities" gene wild Peruvian potato, accidentally caught in the plant due to cross-breeding. Genetic engineering makes it possible to avoid such a risk of accidental introduction of new genes, All used "components" are carefully selected and validated. Among certain advantages that have arisen due to the “Lenope phenomenon”, we can mention at least mandatory testing of all new varieties (both GMOs and traditional breeding) for the content of glycoalkaloids in them.

unknown folk source
The most common "potato threat" is considered solanine, which "forms in green potato" and the next phytomeat is devoted to him, the consequences of his use, as well as methods of salvation from it. Since the information on the Internet is very fragmented, and even it is simply not there - I tried to write in such a way that I got an exhaustive tutorial

Recently, along with such stable expressions as “antioxidants” or “anti-nutrients”, the concept of phytoalexins is used in world phytochemistry. In this way, researchers are trying to reduce all herbal components that have antimicrobial and often antioxidant properties of substances synthesized de novo plants, which quickly accumulate in the areas of infection with pathogens and are a plant "immune response." As a rule, these substances are inhibitors of a wide spectrum of action with a very diverse chemical structure and specific features inherent in certain plant species. In general, phytoalexins are divided into several classes: terpenoids, glycosteroids, alkaloids. Many researchers are in favor of expanding the definition of "phytoalexin" and the inclusion in this concept of all phytochemical components,
Solanine
In English, the Solanaceae family (the same family, which includes our beloved potatoes, tomatoes, eggplants, sweet peppers, but also tobacco, mandragora, belladonna, henbane and dope) is also called nightshades, literally "night shadows." The appearance of this name is sometimes attributed to the fact that even the ancient Romans actively used the plants of this family to prepare various poisons. This makes sense, since in my opinion, the most powerful weapon of plants is their alkaloids. In essence, alkaloids are common chemical nitrogenous compounds, most often in plants acting as a secondary metabolite. In plants of the Solanaceae family, the most bioactive are the so-called. tropane alkaloids (scopolamine, atropine, hyoscyamine, nicotine) and precisely because of their bioactivity, these compounds have been used most often since ancient times as poisons (belladonna or henbane poison, the psychoactive effect of dope, mandrake and tobacco).
Note : it is scopolamine, the alkaloid contained in such plants as scopoly, belladonna, henbane, dope, at the beginning of the 20th century was proposed as a “truth serum”, a disinhibiting effect was caused by the introduction of so-called state of "twilight consciousness". This, of course, is not quite the effect, but nevertheless ...
Solanine is a class of alkaloids of plants of the Solanaceae family, which is mostly present in potatoes.
Note : it is often possible to find a statement that solanine is also found in tomatoes. This is not true. Tomatoes contain “something similar” - an alkaloid tomatin (or lycopersicin). It is tomato and its derivative, tomato, that play a major role in confronting a tomato in front of a fungal, microbial, insecticidal (because of the presence of tomato, the tomato does not take on the Colorado potato beetle) (well, of course, from snails and goats ). Therefore, most of the alkaloid is concentrated in the leaves and inflorescences, approximately 1/5 falls into the fruit (and when ripe it gradually decreases to a certain indicator depending on the variety). Why I decided to mention tomato. Firstly, it is almost always confused with solanine, although tomato is ten times less toxic than solanine (LD 50oral intake of about 500 mg / kg body weight). And secondly, it illustrates what every phytosterol aspires to "- when in contact with cholesterol in any product, it instantly binds it into an insoluble complex and removes it from the body without digestion. In general, if the hands reach the phyto-consideration of tomatoes, I will tell you more about this interesting alkaloid.
We return to the solanine. This glycoalkaloid was discovered as early as 1820 in Black Nights ( Solanum nigrum ) berries , after which it was named. It belongs to the chemical family of saponins. I once wrote about saponins in one of my banana notes:
Saponins are substances that are glycosidic compounds with a steroid (C 27 ) or triterpenoid (C 30 ) (139) sapogenic core with one or more carbohydrate side chains. Due to their amphiphilic character and surface-active properties, saponins are excellent foaming agents, forming a very stable foam.
Where does solanine come from in potatoes
Under natural conditions, the cycle of alkaloids constantly occurs in potatoes, i.e. during the growth of the plant, tens and hundreds of different compounds are biosynthesized. Naturally, there are many alkaloids among them, including the already mentioned solanine. Solanine together with its related compound, haconin, is synthesized in potatoes from acetyl-coenzyme A and cholesterol. The scheme by which all this happens can be seen below (the image is clickable).

Detailed scheme of the cycle of solanine in potatoes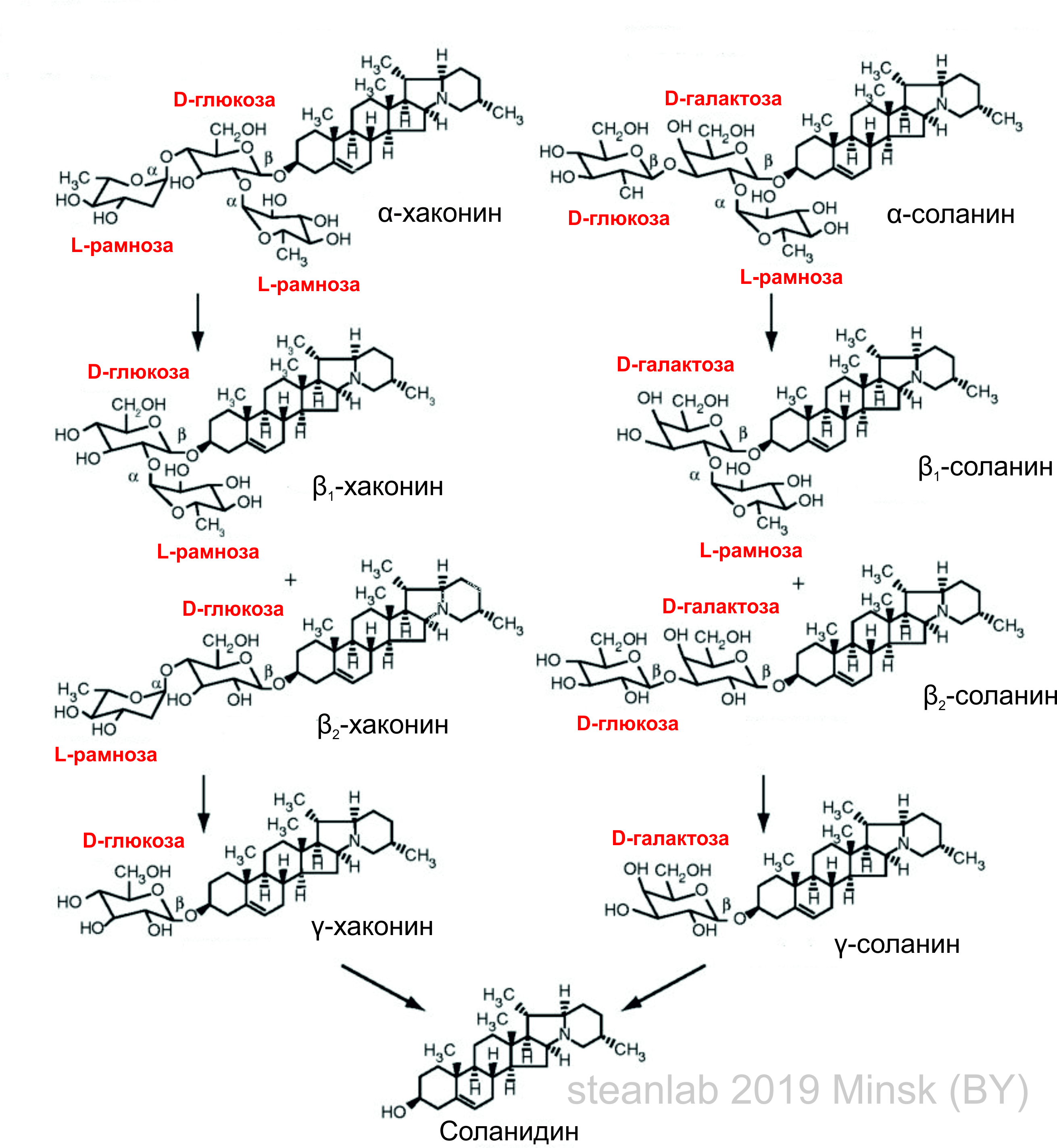

The mentioned alkaloids are constantly synthesized as a kind of “depot protection” against insects, fungi, microorganisms and herbivores.
Minute lyrics. Here I look at these biosynthesis schemes and I understand that chemistry should strive for just such a type. Necessary compounds, peptides, etc. - synthesized in plants, not in flasks. And the chemist's laboratory is some kind of experimental field, not a laboratory room in which you can hang an ax. I don’t know how quickly my dreams will come true, but my favorite D. Cronenberg, in his Existency ( eXistenZ ), was already growing a weapon inside a living reactor ...
OK, continue. The greatest accumulation of protective alkaloids is observed in those parts of the plant that are most at risk of being eaten (ie, in parts located above the ground) - in the leaves, stems, shoots and inflorescences. Under normal (= "greenhouse") conditions, only a small amount (relative to the amount in the remaining parts) of the alkaloids is synthesized in tubers.
But the situation changes dramatically when the tuber is pulled out of the ground, they begin to be transported and stored in anthropogenic conditions. At the beginning of the article I wrote about phytoalexins, as substances that start to generate plants when they get into unfavorable conditions (uncomfortable) environmental conditions or are exposed to damage (mechanical, fungal, bacterial, etc.). The response of a plant to such factors is reflected in an increase in the production of glycoalkaloids (sometimes several times). Actually, the entire mechanism of biosynthesis works only for “war”.
The most common "folk" sign of the presence of high solanine content is the green color of the potato.

IMPORTANT : although the green color of the potato, which was exposed to light radiation, is only the color of chlorophyll (which, by the way, is actively used as a dye in food products, like cheese , and completely harmless). Solanine, which is synthesized in such tubers (after all, a bright light = “tuber on the surface” => include protection against eating = “synthesize killer doses of solanine and hakonin) in general does not have color and it is impossible to notice it with the naked eye. As in the case of a banana - this is just a color indication of a possible (but not mandatory) increase in the level of glycoalkaloids. Those. Each of the processes (accumulation of solanine and greening of potatoes) can quite do without the other.
By the way, Ukrainian researchers experienced this inconsistency earlier than others and began to create a biosensor on for the rapid detection of glycoalkaloids, which exploits the anticholinesterase activity of glycoalkaloids. True, despite the fact that more than 10 years have passed, "things are still there." So - we work in the old manner.
Continuing the theme of greening, we can safely say that “and my grandmother was right again,” when she advised me to cut the peel of green potatoes into white flesh. Indeed, in potato tubers from 30 to 80% of the solanine / hakonin fraction content falls on the peel and the layer of pulp under it is about 7-10 mm (i.e., on the skin, bark and vascular ring). To better understand what to trim - below is a graphic illustration.

In addition to sunlight, fungal diseases (all kinds of “rot”, etc.), as well as damage to tubers during harvesting and transportation, also play the role of aggressive external factors that lead to “corned beef”. By the way, a small life hack. Saponins are generally known for having a very bitter taste, which frightens herbivores even in small doses. This is completely true for solanine. Those. if a bitter taste is observed in a green potato, such a vegetable is only for ejection (it is absolutely impossible to feed even cattle). It is bitterness that is considered the most reliable criterion for the presence of solanine (although I would partially argue, since some short-chain peptides formed during the incomplete enzymatic hydrolysis of potato proteins and which can be foundin raw potatoes, they also give a bitter taste, but these are chemical particulars, in general - bitter potatoes will definitely be harmful to the body).
UPD : the Solairw user , in his commentary, touched upon the fact that I initially missed from attention, about “potatoes with sprouts”, i.e. sprouted potatoes, "about the danger of which is also often said."

When storing potatoes, the process is so-called. aging, during which some biochemical processes in the tuber take place slowly but surely. If the external conditions (temperature, humidity, light) are within the physiological "potato" ratethen there is no noticeable deterioration in the quality of the tuber. Yes, there is a gradual decrease in starch and total sugar, yes, there is a proteolysis of patatin potato protein. But, for example, the content of antioxidant components practically does not change, and all protein compounds are inactivated during heat treatment (during cooking / frying). A completely different situation arises if, due to improper storage conditions, the tubers are exposed to cold / heat shock (stress) or excessive light exposure. As a response to such factors unfavorable to the tuber, the defense mechanism is activated and the potatoes begin to grow green and germinate. As a rule, this is accompanied by enhanced biosynthesis of solanine / haconin glycoalkaloids, and they are localized mainly in those same shoots (read “shoots take a hit”). What does this mean for the average consumer? And all the same. In principle, everything said in the article is equally applicable for sprouted potatoes and for non-sprouted. It is just worth removing the sprouts together with the greened skin, thus minimizing possible contact with poisonous alkaloids and then just boil / fry the potatoes as if nothing had happened. Of course, such potatoes have slightly deteriorated taste (less starch / sugars), but then the glycemic index will be lower. Most people consider sprouted potatoes to be harmful and immediately discarded, but I note that it is much less harmful than not sprouted, but damaged by various kinds of rot. The fact that the problem really exists is confirmed by the attention paid to such a respected organization, as the US Department of Agriculture (the nutrient base of which I constantly use when writing my articles). Every year, hundreds of tons of sprouted potatoes are thrown into the United States, which is still quite possible to be eaten without much harm by simply processing it correctly. In the form of confirmation under a spoiler a piece of the methodical "potato" recommendations of the USDA.
Excerpt from the USDA manual (Ministry of Agriculture of the USA) on the safe use of potatoes

Due to the high prevalence of potatoes (and therefore the availability of skills to work with them among consumers), cases of solanine poisoning are quite rare under normal conditions (i.e., when there is enough food). The danger manifests itself in areas of humanitarian disasters, armed conflicts, etc. where food shortages. But even in such cases, the removal of 10–15 mm of peel from each tuber will relieve the “gourmet” from a large dose of glycoalkaloids (the rest will be removed by heat treatment, but more on this below).
Biological effects and symptoms of poisoning
About what solanin arises from and why he does it, I told. It would be logical to assume that the reason for its deadly harm should be explained. This will be true for both saponins and other surfactants (surfactants). In their action, glycoalkaloids put pressure on two diseased sites - they destroy cell membranes and inhibit the enzyme acetylcholinesterase (even in rats they induce destruction of the liver, but “this is not accurate”).
The mechanism of action on the cell membrane is shown in the picture (the image is clickable)

By their poisonous effect on the cell membrane, glycoalkaloids are required to form stable stoichiometric (1: 1) complexes with cholesterol, which in turn is one of the most important cellular substances (and not just “cholesterol plaques”, as someone might think). After entering the body, the alkaloid part (aglycone) of solanine reversibly binds to sterols, which form a double lipid layer (read about this layer in the last banana note , and a reminder picture under the spoiler)
Scheme of the double lipid layer, which destroy glycoalkaloids

When the density of glycoalkaloids in the membrane reaches a certain level, the “sugar” carbohydrate heads of our “potato poisons” will begin to electrostatically attract each other and form already stable and completely irreversible glycoalkaloid-sterol complexes. In the place of sterols lost to the organism, sterols from the inner side of the double-lipid layer destroyed by alkaloids will be pulled from the inner side (to “mend” the gap that has formed) ”. As a result, roughly foam bubbles form in the double lipid layer, its integrity and performance are impaired. The cell in which the membrane is destroyed - perishes. For example, when glycoalkaloids enter the intestine and disruption of the double lipid layer of the membrane of epithelial cells, macromolecules of everything else (including the alkaloids themselves) begin to flow into the blood. Actually, glycoalkaloids destroy one of the main cellular protective barriers of the body. In addition, the damaged membrane changes its potential and reduces the active transport of sodium ions, which affects, for example, cardiac activity. Solanine is able to "open" potassium channels of mitochondria, increasing their membrane potential. This, in turn, leads to the transportation of Ca2+ from mitochondria into the cytoplasm, and such an increase in the concentration of calcium ions in the cytoplasm causes cell damage and apoptosis .
The second poisoning effect is the inhibitory effect on the enzyme acetylcholinesterase . Here is what Wikipedia says about this :
Acetylcholinesterase inhibitors (organophosphate insecticides, DFP, sarin, soman and V-gases, fasciculin, and some other snake venom peptides) are powerful toxins whose effects on the human body usually lead to death from cramps in the respiratory muscles.As some probably know from school biology (Soviet times) acetylcholine is an important neutrotransmitter in the human body, and it is he who is responsible for transmitting electrical signals from neurons to muscles. Acetylcholine is released by neurons and acts on the sheath of muscle fibers, causing depolarization and contraction (or relaxation, to whom that is more pleasant). In order for the muscle to be reduced again, it is necessary that the released acetylcholine is already cleaved by the enzyme acetylcholinesterase. If there is no such enzyme (or it is “frozen” = inhibited), then acetylcholine gradually accumulates in the synapses and muscles, completely disrupting the transmission of signals from the nerves to the muscles. In principle, the action is simple and quickly fatal. There are truth studies that suggest thatbutyrylcholinesterase , which may be responsible for cell growth. But what kind of cell growth can we talk about when nerve signaling is disturbed ...
A rather unpleasant effect is described in the article . The authors established an epidemiological correlation between the congenital malformations of the fetal nervous system (anencephaly and vertebral artery cleft) in pregnant women and the use of potatoes with increased glycoalkaloid content in women.
Main symptomssimple poisoning with solanine are mainly gastrointestinal manifestations, i.e. vomiting, diarrhea, abdominal pain (therefore, there are cases when poisoning with solanine was taken for gastroenteritis) + in acute poisoning, symptoms typical for poisoning with ARS with acetylcholinesterase inhibitors can also be added (as well as fever, rapid pulse, low blood pressure, rapid breathing, etc.). Estimates of LD 50 toxicity vary, it is believed that a dose of 2–5 mg / kg body weight causes toxic manifestations, and 5–10 mg / kg can be fatal. There is even a tacit guide that recommends the concentration of glycoalkaloids = <200 mg / kg wet weight when developing new potato varieties.
There is a story that the mention of solanine was found in many textbooks for terrorists seized by the FBI in Afghanistan, and Islamic terrorists can use solanine as a weapon of mass destruction. In the seized benefits described available methods for obtaining poisons.In principle, not so far from the truth, it remains only to learn to “ask” potatoes to generate solanine for the order ...
Note: it will be wrong to show only the dark side of power. Therefore, I note that some acetylcholinesterase inhibitors are even synthesized artificially for use in the treatment of Alzheimer's disease, dementia with Levi's calves, myasthenia, can be used as an antidote for anticholinergic poisoning (cyclodol, dimedrol, taren). Comparatively recently studies have been conducted.on cell cultures, which showed high efficacy (comparable to the anticancer drug adriamycin) of solanin and hokanin in inhibiting the growth of cancer cells in tumors of the colon and liver in humans. The anti-cancer effect of glycoalkaloids was also observed when using cell cultures of cervical cancer, lymphoma and stomach cancer. Moreover, the use of two or more glycoalkaloids allowed to achieve a stable synergistic effect. There is also a work where it has been shown that the use of haconin reduces the number of metastases in lung cancer and may in the long run create a new direction of chemotherapy.
So how to escape?
Who is to blame, I hope, clearly. But what to do? And to ask permission from the potato to eat it, as in the old days, people asked permission from the tree to cut it down in order to build a house. Moreover, it is better to tell the tuber how to spend the power that you will get from the starch. Whether the silushka will go to good business. And having eaten potatoes, you feel, as you feel, the tuber, as it were understood by you, the test “learn your eloquence for plants”. Treat the potato with respect, and he will not poison you with solanine.
But seriously, it is very likely that the attentive reader has already partly understood from the text of the article how to protect themselves from being poisoned by solanin & hokanin. Firstly, you need to choose potatoes with minimal mechanical damage (it’s not for nothing that people in shops intuitively buy clean, well-groomed potatoes from Israel, and don’t take a Belarusian one, which is not only expensive, but also scratched, potholed, rotten) signs of fungal and bacterial diseases. Secondly, if there are suspicions of the presence of harmful glycoalkaloids - just remove the peel to a depth of 1-1.5 cm (of course, nothing can remain in the end from small potatoes), this will remove up to 80% of the potato alkaloids.
Important!: The popular methods of heat treatment (cooking, frying, baking), on the contrary, have a weak and “with varying success” effect on the content of solanine and its comrades. For example, cooking potatoes reduces the level of α-haconin and α-solanine by only 3.5% and 1.2%, respectively (but at the same time, cooking in a microwave reduces the content by 15%). Deep roasting at 150 ° C does not lead to any tangible changes, significant destruction of glycoalkaloids begins only at ~ 170 ° C, and frying at 210 ° C for 10 minutes results in a loss of 40% of harmful alkaloids (another thing is then there will be such potatoes). Freeze drying or other types of dehydration does not have a significant effect on the content of solanine in general.
Third, the toxicity of alkaloids in food can also be affected by the products in its (food) composition. After all, you can combine them so that solanine and his ilk adsorbed or at least turned into an inactive compound. Glycoalkaloids are generally hard to digest in the body, the presence of fiber (read dietary fiber) interferes with absorption. Folic acid, glucose-6-phosphate and Nicotinamide adenine dinucleotide ( NAD ) bind haconin into non-toxic complexes. The most active form of solanine & haconin is the β-form (see picture above), respectively, all that contribute to the hydrolysis of this type of glycoalkaloids reduces toxicity (and this can be low pH or exposure to mold fungi Aspergillus niger (!!)).
By the way, there is such an interesting direction in food business (to write an article about which Alexey Shukayev long ago asked me ) as food pairing .
FoodPairing is a method for determining combinations of products that are best combined with each other in terms of taste (i.e., having a common flavor component). The approaches currently used combine products in terms of digestion. The method uses HPLC, gas chromatography and other laboratory methods for analyzing food and finding common chemical components for them.
It is obvious that a cook with such skills is not a “graduate of a culinary technical school,” but rather a chemist and even a nanotechnologist (as with the Fugu fish, when people have been learning how to prepare it for years). Although I fully admit that there are cooks without a proper background, but with God's spark, who, not knowing any chemistry and biology there, can, on a whim, prepare "ambrosia and nectar" that grant health and cure for any ailments. Namely, this should, in my opinion, be any food (about functional food look in the banana article ).
And fourth. At the very extreme case I will say that in case of acute poisonings with the mentioned alkaloids, cholinesterase reactivators are introduced into the body.. They contribute to the restoration of cholinesterase activity, providing an antidote effect. In the first stage of poisoning, dipyroxime is used . In case of pronounced disorders of mental activity (lethargy, coma), a centrally acting drug, isonitrosin, is additionally administered . At the third stage of poisoning, the combined use of dipyroxime with isonitrosin is necessary.
On this all, take care of yourself and be careful! And to be fully prepared - read Habr (# phytochemist notes) :-)
PS I can not fail to mention the fact voiced in the comments ( one and two times ) by the user minamoto . Let's call it the phenomenon of " potato Lenope". A variety with this name was introduced to the US market in 1967 and already in 1970, these potatoes had to be massively withdrawn from crop rotation. The reason for this was mass poisoning caused by several times too high content of potato glycoalkaloids (16–35 mg / 100 g fresh). masses of potato varieties Lenope versus 3–18 mg / 100 g of fresh mass in potatoes of other varieties.) Interestingly, the variety was bred using traditional breeding methods (this fact is often used as counter-arguments in conversations with opponents of GMO fiction). some torye researchers, exceptionally high levels of glycoalkaloids were due to "impurities" gene wild Peruvian potato, accidentally caught in the plant due to cross-breeding. Genetic engineering makes it possible to avoid such a risk of accidental introduction of new genes, All used "components" are carefully selected and validated. Among certain advantages that have arisen due to the “Lenope phenomenon”, we can mention at least mandatory testing of all new varieties (both GMOs and traditional breeding) for the content of glycoalkaloids in them.

References
Caldwell, K. A., Grosjean, O. K., Henika, P. R., & Friedman, M. (1991). Hepatic ornithine decarboxylase induction by potato glycoalkaloids in rats. Food Chem. Toxicol., 29, 531–535.
Friedman, Mendel; McDonald, Gary M. (1999). «Postharvest Changes in Glycoalkaloid Content of Potatoes». In Jackson, Lauren S.; Knize, Mark G.; Morgan, Jeffrey N. Impact of Processing on Food Safety. Advances in Experimental Medicine and Biology. 459. pp. 121–43.
Gao, Shi-Yong; Wang, Qiu-Juan; Ji, Yu-Bin (2006). «Effect of solanine on the membrane potential of mitochondria in HepG2 cells and [Ca2+]i in the cells». World Journal of Gastroenterology. 12 (21): 3359–67.
Friedman, Mendel (2006). «Potato Glycoalkaloids and Metabolites: Roles in the Plant and in the Diet». J. Agric. Food Chem. 54 (23): 8655–8681. doi:10.1021/jf061471t. PMID 17090106
Review of Toxicological Literature prepared for Errol Zeiger, PhD, National Institute of Environmental Health Sciences, Submitted by Raymond Tice. Testing Status of Agents at NTP (National Toxicology Program). February 1998
Friedman, M., Henika, P. R., & Mackey, B. E. (2003). Effect of feeding solanidine, solasodine and tomatidine to non-pregnant and pregnant mice. Food Chem. Toxicol., 41, 61–71
Mensinga, T. T., Sips, A. J., Rompelberg, C. J., van Twillert, K., Meulenbelt, J., van den Top, H. J., & van Egmond, H. P. (2005). Potato glycoalkaloids and adverse effects in humans: an ascending dose study. Regul. Toxicol. Pharmacol., 41, 66–72.
Friedman, M., & Levin, C. E. (2008). Review of methods for the reduction of dietary content and toxicity of acrylamide. J. Agric. Food Chem., 56, 6113–6140.
Friedman, M., & McDonald, G. M. (1995). Acid-catalyzed partial hydrolysis of carbohydrate groups of the potato glycoalkaloid a-chaconine in alcoholic solutions. J. Agric. Food Chem., 43, 1501–1506.
Friedman, M., & McDonald, G. M. (1997). Potato glycoalkaloids: chemistry, analysis, safety, and plant physiology. Crit. Rev. Plant Sci., 16, 55–132.
Friedman, M., & McDonald, G. (1999). Postharvest changes in glycoalkaloid content of potatoes. In: L. Jackson, M. Knize (Eds.). Impact of Food Processing on Food Safety, Vol. 459 (pp. 121–143). Plenum Press, New York.
Friedman, M., & McDonald, G. M. (1999). Steroidal glycoalkaloids. In: R. Ikan (Ed.), Naturally Occurring Glycosides: Chemistry, Distribution and Biological Properties (pp. 311–342). Wiley, New York and London.
Friedman, M., McDonald, G. M., & Haddon, W. F. (1993). Kinetics of acid-catalyzed hydrolysis of carbohydrate groups of potato glycoalkaloids a-chaconine and a-solanine. J. Agric. Food Chem., 41, 1397–1406.
Friedman, M., McQuistan, T., Hendricks, J. D., Pereira, C., & Bailey, G. S. (2007). Protective effect of dietary tomatine against dibenzo[a,l]pyrene (DBP)-induced liver and stomach tumors in rainbow trout. Mol. Nutr. Food Res., 51, 1485–1491.
Friedman, M., Roitman, J. N., & Kozukue, N. (2003). Glycoalkaloid and calystegine contents of eight potato cultivars. J. Agric. Food Chem., 51, 2964–2973.
Shih, Y. W., Chen, P. S., Wu, C. H., Jeng, Y. F., & Wang, C. J. (2007). Alpha-chaconine-reduced metastasis involves a PI3K/Akt signaling pathway with downregulation of NF-kappaB in human lung adenocarcinoma A549 cells. J. Agric. Food Chem., 55, 11035–11043.
Laha, M. K., & Basu, P. K. (1983). Biological hydrolysis of glycoalkaloids from Solanum khasianum by a local strain of Aspergillus niger. Int. J. Crude Drug Res., 21, 153–155.
Friedman, Mendel; McDonald, Gary M. (1999). «Postharvest Changes in Glycoalkaloid Content of Potatoes». In Jackson, Lauren S.; Knize, Mark G.; Morgan, Jeffrey N. Impact of Processing on Food Safety. Advances in Experimental Medicine and Biology. 459. pp. 121–43.
Gao, Shi-Yong; Wang, Qiu-Juan; Ji, Yu-Bin (2006). «Effect of solanine on the membrane potential of mitochondria in HepG2 cells and [Ca2+]i in the cells». World Journal of Gastroenterology. 12 (21): 3359–67.
Friedman, Mendel (2006). «Potato Glycoalkaloids and Metabolites: Roles in the Plant and in the Diet». J. Agric. Food Chem. 54 (23): 8655–8681. doi:10.1021/jf061471t. PMID 17090106
Review of Toxicological Literature prepared for Errol Zeiger, PhD, National Institute of Environmental Health Sciences, Submitted by Raymond Tice. Testing Status of Agents at NTP (National Toxicology Program). February 1998
Friedman, M., Henika, P. R., & Mackey, B. E. (2003). Effect of feeding solanidine, solasodine and tomatidine to non-pregnant and pregnant mice. Food Chem. Toxicol., 41, 61–71
Mensinga, T. T., Sips, A. J., Rompelberg, C. J., van Twillert, K., Meulenbelt, J., van den Top, H. J., & van Egmond, H. P. (2005). Potato glycoalkaloids and adverse effects in humans: an ascending dose study. Regul. Toxicol. Pharmacol., 41, 66–72.
Friedman, M., & Levin, C. E. (2008). Review of methods for the reduction of dietary content and toxicity of acrylamide. J. Agric. Food Chem., 56, 6113–6140.
Friedman, M., & McDonald, G. M. (1995). Acid-catalyzed partial hydrolysis of carbohydrate groups of the potato glycoalkaloid a-chaconine in alcoholic solutions. J. Agric. Food Chem., 43, 1501–1506.
Friedman, M., & McDonald, G. M. (1997). Potato glycoalkaloids: chemistry, analysis, safety, and plant physiology. Crit. Rev. Plant Sci., 16, 55–132.
Friedman, M., & McDonald, G. (1999). Postharvest changes in glycoalkaloid content of potatoes. In: L. Jackson, M. Knize (Eds.). Impact of Food Processing on Food Safety, Vol. 459 (pp. 121–143). Plenum Press, New York.
Friedman, M., & McDonald, G. M. (1999). Steroidal glycoalkaloids. In: R. Ikan (Ed.), Naturally Occurring Glycosides: Chemistry, Distribution and Biological Properties (pp. 311–342). Wiley, New York and London.
Friedman, M., McDonald, G. M., & Haddon, W. F. (1993). Kinetics of acid-catalyzed hydrolysis of carbohydrate groups of potato glycoalkaloids a-chaconine and a-solanine. J. Agric. Food Chem., 41, 1397–1406.
Friedman, M., McQuistan, T., Hendricks, J. D., Pereira, C., & Bailey, G. S. (2007). Protective effect of dietary tomatine against dibenzo[a,l]pyrene (DBP)-induced liver and stomach tumors in rainbow trout. Mol. Nutr. Food Res., 51, 1485–1491.
Friedman, M., Roitman, J. N., & Kozukue, N. (2003). Glycoalkaloid and calystegine contents of eight potato cultivars. J. Agric. Food Chem., 51, 2964–2973.
Shih, Y. W., Chen, P. S., Wu, C. H., Jeng, Y. F., & Wang, C. J. (2007). Alpha-chaconine-reduced metastasis involves a PI3K/Akt signaling pathway with downregulation of NF-kappaB in human lung adenocarcinoma A549 cells. J. Agric. Food Chem., 55, 11035–11043.
Laha, M. K., & Basu, P. K. (1983). Biological hydrolysis of glycoalkaloids from Solanum khasianum by a local strain of Aspergillus niger. Int. J. Crude Drug Res., 21, 153–155.
