How low-calorie food affects aging
- Transfer
Anti-Aging and Calorie Reduction Mechanisms: Data from Genetically Modified Animals.
It is well known that caloric restriction (salorie restriction, CR) increases longevity and suppresses various pathophysiological changes. CR inhibits growth hormone / insulin-like growth factor and mTORC1 signaling, activates sirtuin and enhances mitochondrial redox regulation. But the exact mechanisms are under discussion. In this review, we will discuss the mechanisms of CR, using data from animal studies that have been genetically modified according to recent advances in molecular and genetic technologies, in terms of the adaptive response hypothesis proposed by Holliday in 1989. We will also explain the positive actions of CR, which are classified according to whether they are valid under nutrition or fasting.
In 1935, it was described that CR increases life expectancy in rats [1]. CR, also known as dietary energy restriction or restriction, is widely used in aging studies as a strong and simply reproducible dietary manipulation to prolong life. The effect of CR was observed in several species, from yeast and nematodes to mammals. In mammals, it was mainly studied using rodents, in which CR suppresses various age-related pathophysiological changes and prolongs the average and maximum lifespan. However, the beneficial effects of CR disappear in certain strains and / or conditions. A recent review describes these limitations in detail [2]. The extent to which CR has beneficial effects depends on factors such as rodent species, strains, and the timing of onset of CR. In general, however, long-term CR, started at a young age suppresses age-related pathophysiological changes and prolongs the longevity of different rodents. It is also important that the limitations of individual nutrients (for example, glucose, lipid, protein) without limiting energy do not cause such beneficial effects [3, 4].
More than 20 years ago, it was discovered that Ames dwarf mice that have the Prop1 gene mutation live longer than wild-type mice [5]. This was the first report that a single mutation of a gene or a genetic modification is capable of extending longevity in mammals. According to our information, more than 40 mice and rats with a single gene mutation or genetic modification live longer than wild-type animals. Of these mice and rats, about one third demonstrate suppressed growth hormone (GH) signaling / insulin-like growth factor 1 (IGF1). Because CR also suppresses GH / IGF1 signaling, CR efficiencies can be based on this. Other molecular mechanisms that have been proposed for regulating the beneficial effects of CR include the inhibition of the activity of the mechanistic target of rapamycin complex 1 (mTORC1), the activation of autophagy, activation of NAD + and sirtuin metabolism and increased mitochondrial redox regulation [6, 7]. However, these mechanisms are not fully understood.
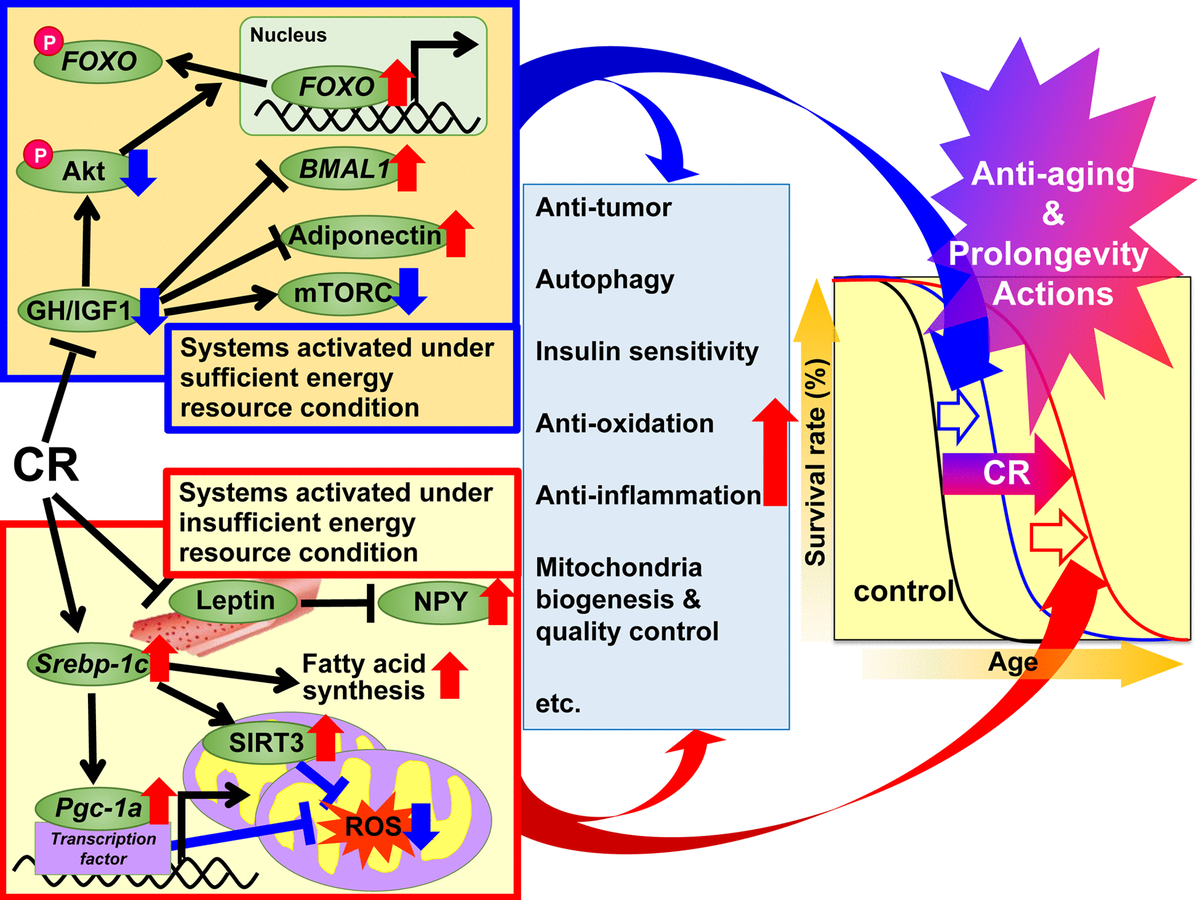
Fig.1. The proposed mechanisms of action of caloric restriction (CR) against aging and to increase the life expectancy based on the adaptive response hypothesis. It is proposed that the CR regulatory mechanisms be divided into two systems. The first system is activated under sufficient energy conditions, when there is an opportunity for free use of energy, and animals grow well, reproduce more and store excess energy in the form of triglycerides (TG) in white adipose tissue (WAT) for later use. This system includes growth hormone (GH) / insulin-like growth factor 1 (IGF1), Akt, FOXO, mTORC, adiponectin, and BMAL1. The second system is activated in conditions of insufficient energy, when there is no benefit from excessive use of energy, and animals inhibit growth and reproduction and use the energy saved to maintain biological function. This system includes the following signaling pathways: SREBP-1c protein, sirtuin (SIRT), PGC-1α protein, mitochondrial reactive oxygen species (ROS), leptin and neuropeptide Y (NPY) .. In animals with CR, these signals act effectively for energy use. Moreover, various signals and / or factors may contribute to the beneficial effects associated with CR, including antioxidant, anti-inflammatory, anti-tumor and other effects of CR to varying degrees in each tissue or organ and thus lead to rejuvenation and longer life. leptin and neuropeptide Y (NPY) .. In animals with CR, these signals act effectively to use energy. Moreover, various signals and / or factors may contribute to the beneficial effects associated with CR, including antioxidant, anti-inflammatory, anti-tumor and other effects of CR to varying degrees in each tissue or organ and thus lead to rejuvenation and longer life. leptin and neuropeptide Y (NPY) .. In animals with CR, these signals act effectively to use energy. Moreover, various signals and / or factors may contribute to the beneficial effects associated with CR, including antioxidant, anti-inflammatory, anti-tumor and other effects of CR to varying degrees in each tissue or organ and thus lead to rejuvenation and longer life.
CR targets and molecular mechanisms
GH, IGF1 and FOXO1 signaling
GH positively regulates the production of IGF1 predominantly in the liver via the GH receptor (GHR). IGF1 acts on the IGF1 receptor, and then phosphorylates Akt, serine / threonine kinase in target cells. Then the phosphorylated form Akt phosphorylates the transcription factors FOXO, promoting nuclear export. Therefore, the suppression of GH / IGF1 signaling transcriptionally increases the expression of several genes activated by FOXO transcription factors.
Several modified mouse species, Ames dwarf, Snell dwarf and GHR knockout (GHR KO), show suppressed GH signaling and have an increased lifespan. These dwarf mice have similar phenotypes with CR mice, including suppressed GH / IGF1 signaling, reduced levels of thyroid hormone, insulin and glucose, lower body temperature and reduced obesity. However, the gene expression profile of the liver differs significantly between GHR KO mice and CR mice [8]. We also reported that part of the gene expression profile in white adipose tissue (WAT) of CR rats is significantly different from that of lifelong dwarf rats carrying the antisense GH transgene [9].
Bonkowski et al. CR reported that it increases insulin sensitivity and increases life expectancy in wild-type mice, but not in GHR KO mice [10]. Therefore, they suggested that the effect of extending the lifetime of a CR depends on the suppression of GH / IGF1 signaling. In dwarf mice and dwarf rats carrying the antisense GH transgene, CR further increased their lifespan [11, 12]. These data suggest that anti-aging actions and prolonged CR can be regulated both dependent on the GH / IGF1 signal and independently.
FOXO transcription factors in mammals consist of four isoforms, that is, FOXO1, 3, 4, and 6. FOXO1 KO mice (with a knockout of this gene) have an increased CR lifespan, but no anti-tumor effect associated with CR [13]. Conversely, in FOXO3 KO mice, the addition of CR suppressed tumorigenesis, but there was no CR-induced increase in longevity [14]. These differences may be related to the differential activation pattern in the tissues and / or cells of the four isoforms of the FOXO transcription factors induced by CR.
BMAL1 protein is a transcription factor involved in the regulation of the circadian rhythm. In BMAL1 KO mice (with a knockout of this gene), food consumption increased, body weight decreased, and aging phenotypes accelerated. In these mice, CR did not lower insulin levels and IGF1 and did not increase life expectancy. What indicates the participation of BMAL1 in the CR beneficial action and that this beneficial action depends on GH / IGF1 signaling [15].
MTOR alarm
mTOR kinase, serine / threonine kinase, was identified as a target molecule of rapamycin. It forms two separate multi-protein complexes, known as mTORC1 and mTORC2. It is known that mTORC1 is activated by amino acids and growth factors (for example, insulin and IGF1). Activation of mTORC1 promotes protein synthesis through the S6 1 ribosomal protein kinase, fatty acid synthesis through the steroid regulator-binding protein (SREBP) 1, and adipocyte differentiation using peroxisomal proliferator-activated receptors (PPARγ). mTORC1 suppresses autophagy and lysosomal biosynthesis through the transcription factor EB (TFEB). The function of mTORC2 is poorly understood, but it is believed that it includes the enhancement of anabolic actions and the suppression of catabolic actions, as for mTORC1 [16].
Mice that were given rapamycin, a negative regulator of mTORC1, had an increased lifespan for a long period after middle age [17]. Consistent with this finding, transgenic mice with overexpression of TSC1 protein, which negatively regulates mTORC1, live longer than wild-type mice [18]. In addition, mice with knockout ribosomal protein kinase S6 1 and mutant mTOR mice also lived longer than wild-type mice [19, 20].
To our knowledge, the beneficial effect of CR has not yet been studied in mice with a defective mTORC1 function. However, yeast with genetic inhibition of mTOR CR did not increase life expectancy [21]. Autophagy is enhanced by the suppression of mTORC1. In nematodes deficient in autophagy-related genes, CR did not increase lifespan [22]. Based on these data, it is likely that the decrease in mTOR activity and the activation of the autophagic apparatus are associated with a positive effect of CR.
Sirtuins
Sir2 was discovered as a new gene involved in the suppression of transcription in yeast. After that, it was reported that he plays a key role in prolonging life during CR [23, 24]. Seven sirtuin orthologous genes, the Sirt1 sirtuins-Sirt7, have been identified in mammals. Proteins SIRT1, 6 and 7 are mainly localized in the nucleus, SIRT2 in the nucleus and cytoplasm, and SIRT 3, 4 and 5 mainly in the mitochondria. Sirtuins catalyze deacetylation reactions of various proteins, including histones, depending on NAD [25].
Among the seven mammalian sirtuins, SIRT1, 3 and 6 are reported to be involved in age-related pathophysiology and life span regulation [26]. Transgenic mice in which the SIRT1 protein was selectively over-expressed in hypothalamic neurons had a longer lifespan than wild-type mice. [27]. Transgenic female mice in which the SIRT6 protein was overexpressed had a longer lifespan than wild-type mice [28]. In elderly mice, CR increased SIRT6 expression for 6 months and improved renal failure. In addition, while overexpression of SIRT6 suppressed cellular aging by reducing the activity of inflammatory-related transcription factor NF-κB, knockout of SIKT6 accelerated cellular aging [29]. In SIRT3 KO mice, various age-related pathologies were previously observed [30].
Transcription factor NRF2
NRF2 binds to elements of the antioxidant response to induce the expression of target genes in response to oxidative stress and enhances the expression of genes involved in antioxidant and detoxification responses. Under physiological conditions, NRF2 binds to Keap1 in the cytoplasm, where it degrades. Under stress, including oxidative stress, after Keap1 is captured by phosphorylated p62, NRF2 translocates to the nucleus, binds to the antioxidant response elements and activates the transcription of antioxidant genes [32].
Since the expression of NRF2 decreases with aging in rodents, it is assumed that the levels of reactive oxygen species and the various risks of cancer increase. However, CR suppresses the age-related decrease in antioxidant capacity by increasing the expression of genes involved in antioxidation and detoxification. In nematodes, Skn-1, a homologue of NRF2, is indispensable for the action of CR to increase its lifespan. NRF2 knockout mice show a decrease in the expression of genes involved in the antioxidant response and detoxification, resulting in accelerated carcinogenesis. The role of NRF2 in the efficacy of CR was investigated using NRF2 KO mice. The results showed that NRF2 is important for the antitumor effect of CR, but does not participate in the effects associated with longevity and increased insulin sensitivity of CR [33].
Neuropeptide Y (NPY)
In mammals, neurons in the hypothalamic arcuate nucleus feel the energy status of the levels of circulation of hormones. The CR-associated negative energy balance and the subsequent decrease in fat mass increases the circulation of ghrelin and adiponectin levels and decreases the levels of leptin, insulin and IGF1 in the blood. These hormonal changes activate NPY neurons in the hypothalamic arcuate nucleus. Most of these neurons synthesize Agrp protein, weakening the activity of POMC neurons in the arcuate nucleus. Changes in the activity of primary neurons inhibit secondary hypothalamic neurons secreting somatotropin, gonadotropin and thyrotropin-releasing hormone, and activate neurons secreting corticotropin-releasing hormone. This hypothalamic change suppresses GH / IGF1 signaling, thyroid function and reproduction and activates the function of adrenal glucocorticoids. Most of these altered neuron secretion profiles are observed in mice and rats with CR [34].
In NPY KO knockout mice, the addition of CR did not increase the lifespan, caused tolerance to oxidative stress in the liver, and altered the profile of neuronal secretion. However, CR decreased the level of insulin and IGF1 in the blood, increased the level of adiponectin in the blood and the level of corticosterone, and reduced the expression of genes involved in beta-oxidation in the liver. Thus, NPY should be a key factor associated with the GF / IGF1 independent CR efficiencies [35].
Mutation of mitochondrial DNA (mtDNA)
It is believed that the accumulation of mtDNA mutations is one of the key factors of pathogenesis in age-related diseases. PolgA D257A / D257A mice carry a mutation in mtDNA polymerase-gamma and show an earlier development of age-related accumulation of mtDNA mutations and age-related phenotypes in various tissues [36]. In PolgA D257A / D257A mice, CR did not prolong life expectancy, did not affect the accumulation of mtDNA deletion in skeletal muscles, and did not improve heart function, and this contributed to sarcopenia. These data suggest that accumulation of mtDNA mutations may inhibit the beneficial effects of CR [37].
Our new results: adipose tissue remodeling under the influence of CR
Visceral obesity associated with diabetes, hyperlipidemia, and hypertension, collectively known as “metabolic syndrome”, is a known risk factor for the development of atherosclerotic diseases associated with the development of life, including myocardial infarction and cerebral infarction. Adipose tissue, originally considered only related to energy function, has recently been described as an endocrine organ that secretes various biologically active molecules called adipokines. Large adipocytes that accumulate triglycerides (TG) excessively increase the secretion of inflammatory adipokines, including tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), and reduce the secretion of adiponectin compared to small adipocytes that accumulate less TG. These adipokine secretion profiles are directly involved in age-related pathologies, including insulin resistance, hypertension and atherosclerosis [38]. In addition, it has recently been reported that adipose tissue and adipokines are key players in regulating longevity. For example, mice with insulin receptor knockout in adipocytes showed a decrease in obesity, an increase in mitochondrial biogenesis, and a longer lifespan than wild-type mice [39]. Transgenic mice with overexpression of adiponectin in the liver showed greater survival than control [40]. The transcription factors PPARγ and CCAAT / enhancer-binding proteins α (C / EBPα) and β (C / EBPβ) are involved in adipocyte differentiation. Mice with the decomposition of the C / EBPβ gene in the C / EBP locus showed enhanced mitochondrial biogenesis and longer lifespans [41]. In contrast, PPARγ KO knockout mice had shorter lifespans than control ones [42].
CR is reported to increase the active form of adiponectin in mice of any age. This CR-associated regulation of adiponectin is dependent on GH / IGF1 signaling [43, 44]. We analyzed the CR-associated change in chronological order and obtained the following results. CR increased the expression of genes and / or proteins involved in fatty acid biosynthesis (FA) and mitochondrial biogenesis in adipose tissue after the early phase of CR. A CR-related change occurred more predominantly in adipose tissue than in other tissues or organs. After that, morphological changes occurred, including adipocyte size reduction and metabolic changes in the liver [45]. To clarify CR-related metabolic changes in adipose tissue that occurred independently of the GH / IGF1 signal, we then compared the gene expression profile for CR in adipose tissue of wild-type rats with transgenic rats, which were given ad libitum (AL). Our results showed that CR enhances the expression of genes involved in the biosynthesis of fatty acids, in particular, in the main transcription factor of the biosynthesis of fatty acids, the regulatory genes of SREBP-1, independently of GH / IGF1 [9].
Therefore, we then compared the effect of CR with various parameters, including the lifespan between the SREBP-1c KO knockout mice and the wild-type mice. SREBP-1c KO mice had a slightly shorter lifespan than wild-type mice. In wild-type mice with an increased lifespan, CR increased the expression of proteins involved in fatty acid biosynthesis and mitochondrial biogenesis, and suppressed oxidative stress. Most of these changes were observed mainly in adipose tissue, and not in other tissues. In contrast, CR-associated life extension and changes in adipose tissue were not observed in SREBP-1c KO mice. PGC-1α is reported to be a key regulator of CR-induced mitochondrial biogenesis [46]. We observed that SREBP-1c binds to the promoter of the Pgc-1α gene, suggesting that SREBP-1c directly regulates the transcription of Pgc-1α [47]. Moreover, the results of the analysis of the proteome of adipose tissue showed that CR activates the pyruvate / malate cycle [48]. Indeed, it has been reported that CR activates de novo biosynthesis of fatty acids in adipose tissue, but not in the liver [45]. These data show that SREBP-1c KO mice cannot effectively use fats under CR conditions. Thus, adipose tissue can not only function as a tissue for energy storage, but can also play the role of converting glucose to a more energy-intensive fatty acid through SREBP-1c under CR conditions. but not in the liver [45]. These data show that SREBP-1c KO mice cannot effectively use fats under CR conditions. Thus, adipose tissue can not only function as a tissue for energy storage, but can also play the role of converting glucose to a more energy-intensive fatty acid through SREBP-1c under CR conditions. but not in the liver [45]. These data show that SREBP-1c KO mice cannot effectively use fats under CR conditions. Thus, adipose tissue can not only function as a tissue for energy storage, but can also play the role of converting glucose to a more energy-intensive fatty acid through SREBP-1c under CR conditions.
Discussion of CR in terms of adaptive response hypothesis
In 1989, Holliday explained the effects of anti-aging and increased CR lifes from an evolutionary point of view on organisms that developed adaptive response systems to maximize survival during periods of food shortages [49, 50]. Based on this evolutionary point of view, we have divided the beneficial actions of CR into two systems; “Systems activated under sufficient conditions of energy resources” and “systems operating under insufficient conditions of energy resources”. The first is activated in a natural environment that provides animals with free use of energy by providing abundant food. In other words, when there is a lot of food for free use of energy, animals grow well, reproduce more and retain excess energy as TG in adipose tissue for later use, but not enough to make them obese. The second system is activated in a natural environment, which does not allow free use of energy due to food shortages. In other words, when there is no benefit from the free use of energy, animals suppress growth and reproduction and use the energy saved from growth and reproduction to maintain their biological function. Adaptation to natural environmental changes is a top priority for animal survival. animals inhibit growth and reproduction and use the energy saved from growth and reproduction to maintain biological function. Adaptation to natural environmental changes is a top priority for animal survival. animals inhibit growth and reproduction and use the energy saved from growth and reproduction to maintain biological function. Adaptation to natural environmental changes is a top priority for animal survival.
Based on the adaptive response hypothesis and the recent findings mentioned above, we propose a set of mechanisms for CR beneficial actions.
Since the experimental conditions of CR can mimic insufficient energy conditions, we have assumed that CR suppresses “systems activated under sufficient energy conditions” and activates “systems activated under insufficient energy conditions” and additively induces actions against aging and to increase the lifespan. The first set of systems includes signals GH / IGF1, FOXO, mTORC, adiponectin and BMAL1, and CR, apparently, suppresses these anabolic reactions. The second set of systems includes SREBP-1c / mitochondrial, SIRT and NPY signaling, and probably CR activates these reactions for optimal utilization of insufficient energy resources. Moreover, various signals and / or factors may contribute to the anti-aging and life-prolonging actions of CR to varying degrees with antioxidant, anti-inflammatory,
Regarding dietary intervention paradigms, not only CR was applied, but also intermittent energy restriction (IER) and nutrition time restriction (TRF) [2]. IER usually includes fasting every other day or 2-3 days a week. TRF, which is more popular in obesity studies than biogeronology studies, generally implies restricting access to nutrition (high in fat) for several hours a day. The beneficial effects caused by IER or TRF are partially similar to those caused by CR. However, to our knowledge, no studies used rigorous research plans, including nutritional schedules, to compare three dietary interventions. Therefore, comparative studies of CR, IER and TRF may be required in the future.
Perspectives
Studies using monkeys show that the beneficial effects of CR can also occur in humans and in other mammals [51]. Current CR research focuses on two topics, that is, the identification of the molecular mechanisms of CR, as well as the development of mimetic drugs CR. We believe that the development of new drugs acting as CR can be difficult without understanding the molecular mechanisms of CR. The development of such drugs that are applicable to humans requires further research on the molecular mechanisms of CR, especially in primates. In this report, we propose to classify and discuss the molecular mechanisms of the beneficial effects of Cheka, depending on whether they work in conditions of rich or insufficient energy resources. Further studies on the molecular mechanisms of the beneficial effects of CR should also take into account The extent to which the involved signals / factors contribute to antioxidant, anti-inflammatory, anticancer, and other actions of CR in each tissue or organ, and thereby lead to rejuvenation and increased longevity. Studies of genetically modified animals with a focus on one of the two systems mentioned above show differences in the extent of CR-induced effects in mice of different origin and those that compare the beneficial effects of CR with IER or TRF factors, will help clarify not only further molecular mechanisms of CR, but also those related to longevity. and thereby lead to rejuvenation and increased longevity. Studies of genetically modified animals with a focus on one of the two systems mentioned above show differences in the extent of CR-induced effects in mice of different origin and those that compare the beneficial effects of CR with IER or TRF factors, will help clarify not only further molecular mechanisms of CR, but also those related to longevity. and thereby lead to rejuvenation and increased longevity. Studies of genetically modified animals with a focus on one of the two systems mentioned above show differences in the extent of CR-induced effects in mice of different origin and those that compare the beneficial effects of CR with IER or TRF factors, will help clarify not only further molecular mechanisms of CR, but also those related to longevity.
Prepared by: Alexey Rzheshevsky.
A source:
Hoshino S, Kobayashi M, Higami Y. Mechanisms of caloric restriction restriction: evidence from studies of genetically modified animals. Aging (Albany NY). 2018 Sep 16.
