Guide to electrical materials for all. Part 6
- Tutorial
Continuing guidance on electrical materials. In this part, we continue to disassemble dielectrics completely synthetic in origin. Ie all known plastics. In this part: carbolite, getinaks, textolite.

Welcome to CAT (TRAFFIC)
Available natural materials were widely used, but with the development of technology it became increasingly clear that natural materials are sometimes complete shit. There is a wide range of properties, susceptibility to rotting, difficulties in extraction - therefore, searches for artificial substitutes have been and are being conducted all the time. The appearance of synthetic materials is not only a technical revolution, but also an economic, political one. You no longer need colonies to cover your rubber needs. Equipping your soldier has become easier several times. In this section - the materials obtained "from scratch", and not an attempt to improve the natural, as in the previous section.
Many of these materials are polymers - materials with long molecules, consisting of simple bricks of the same type - monomers. Polymers can be divided into two large groups according to their behavior during heating; these are thermoplastics and thermoset plastics. Thermoplastics melt when heated, thermoset plastics decompose when heated.
Accordingly, a mountain of old plastic toys from thermoplastics can be melted down into a new product, and a mountain of old products from thermoset plastics cannot be recycled this way.
The polymer may consist of pure monomer, and may also contain a co-polymer, which is embedded in the structure of the molecule. For example, there are two monomers: A and B. A polymer molecule from pure A will look like this:
...- АА А А А А А А А А А А А А А А А А А…
A polymer molecule from copolymers A and B may look like this:
...- A-B-A-B-A-B-A-B-A-B-A-B- ...
Or even this way:
...- A- A-B-B-A-B-B-B-A-A-B-B- ... The
introduction of the copolymer allows you to change the properties of the plastic. An example is polystyrene and ABS plastic. Polystyrene is a transparent fragile plastic, the introduction of an acrylonitrile copolymer and the introduction of an additive from polybutadiene results in a shock-resistant plastic.
Sometimes, polymer stereoregularity may additionally be indicated. Suppose we have a monomer -G-, which can get up into the polymer chain "upside down" -L-. A polymer in which the chain of monomers are haphazardly called atactic:
...- L-G-L-G-LLL-G-G-L-G- ...
If in a polymer all unsymmetrical units look in one direction, such a polymer called isotactic:
...- LLLLLLLLLLLL- ...
If they alternate in a polymer, then such a polymer is called syndiotactic:
...- L-G-L-G-L-G-L-G-L-G-L-G- ...
Usually, stereoregularity affects the material properties of the material insignificantly, so it is not indicated.
Polymers, due to their structure of long molecules, have some common
properties that should be considered more closely.
1. Polymers do not have a clear phase transition temperature, such as metals. They are like caramel, soften with increasing temperature, turning into a viscous liquid. Therefore, for polymers, the “melting point” is the temperature at which the viscosity of the polymer already allows it to flow, but this does not mean that it is solid up to this temperature.
Glass transition temperature- is the temperature below which the polymer from a highly elastic state goes into a glassy state, with increasing hardness and brittleness. Imagine chewing marmalade - at room temperature, it is in a highly elastic state. If it is cooled below the glass transition temperature in the freezer, the marmalade can be broken, and the fragments will be as if from glass.
The maximum working temperature is the temperature at which the polymer can work for a long time, without significant changes in its properties. Often, as the temperature rises, the creep of the polymer increases, and therefore, at the maximum operating temperature, the strength properties decrease.
The indicated temperatures may differ in the determination even for the same sample, with different determination methods.
2. Polymers are subject to aging and degradation. Factors that accelerate the aging process of the polymer are radiation, ultraviolet radiation, high temperature, aggressive environment. Different polymers in different degrees of aging, in addition, various additives can reduce the rate of destruction of the polymer. Thus, a nylon tie on a silicone hose with hot water for a couple of years will lose elasticity and become brittle, while the silicone hose will still be soft and flexible.
Only a very small amount of plastics tolerate long-term heating above 100 ° C - fluoroplastic-4, Kapton, peek, silicones. In all other cases, the higher the operating temperature, the faster the aging and destruction processes in the polymer.
3. Polymers are permeable to gases and some solvents. Gas molecules are very small (the smaller the atomic mass, the smaller the size of the atom, the nastiest hydrogen in this regard, it squeezes even through the metals.) Therefore they can gradually penetrate the extensive molecular network of plastic. To prevent this process, the polymer surface is coated with a metal layer. Pay attention to this when opening the food packaging. Metallization in the package serves this purpose - do not miss the oxygen to the product. Metal-plastic pipes contain a layer of aluminum with the same purpose - to prevent the penetration of oxygen into the coolant, it causes corrosion.
Phenol-formaldehyde resins, as is easy to understand from the name, are products of phenol and formaldehyde polycondensation. Polymer molecules form a branched three-dimensional structure, which determines the mechanical properties - hardness.
Below we consider only phenol-formaldehyde plastics - phenoplasts . Urea-formaldehyde, melamine-formaldehyde plastics - aminos , we will not consider, their basic properties are identical, the processing methods are the same, the difference is only in strength, color.
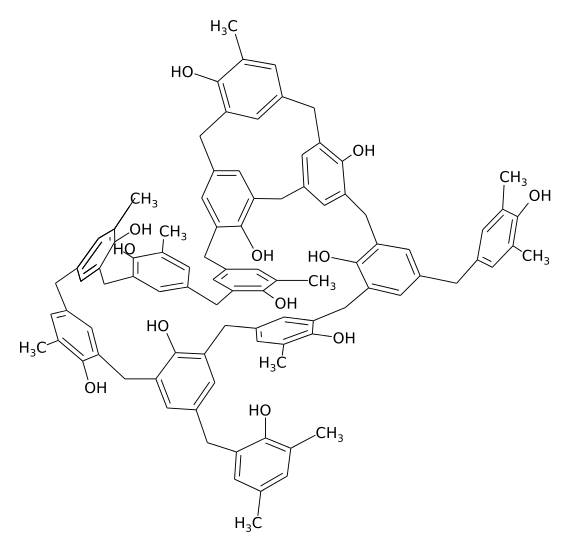
The chemical structure of bakelite (piece for example) Polymers with such an extensive disordered structure are usually hard and brittle. The author of the picture is Dirk Hünniger, taken from Wikipedia.
Leo Bakeland, the American chemist from Belgium, opened the polycondensation process.
origin. He called the new material obtained during the curing of the resin - Bakelite.
In the USSR, a similar material was called "carbolite" - from carbolic acid, the
old name for phenol.
Examples of the use of phenol-formaldehyde resins:
It is a solid heat-resistant plastic. If you take any device
assembled before 1950, then almost all plastic parts in it are carbolite.

Various products from carbolite - box, socket. Plug, voltmeter case, jacks, knobs adjustment.
Products are obtained both by pouring into molds and (more often) by pressing the resin powder with a filler into metal molds with heating. When heated, the polymerization process, which has already begun partly during the production of the powder, ends, but since the powder is squeezed under pressure at the moment in the mold, the appearance of the final product repeats the shape. A serious disadvantage of this method is that it takes time, which the product must spend in the form in order to gain strength enough to open the form without breaking, therefore in many tasks bakelite is displaced by thermoplastic materials, the molding machine can produce products of a given shape much faster.
A little about the process will tell this American advertising video of the last century, appreciate the enthusiasm with which they talk about the new material.

The body of the meter is made of carbolite.
Today products made of carbolite are mass produced, but it is not as popular as before, although there are tasks where it is difficult to replace it with something.
Heat-resistant plastic. It can work for a long time at a temperature of up to + 150 ° C. It is a thermoset - it does not melt, but is destroyed by heat. So carbolite cartridge for incandescent bulbs when overheating crumbles, and does not flow to your head.
Resistant to solvents, fuels and lubricants ( fuel and lubricants). Carbolite parts easily work near the car engine, in conditions of heating, contact with oil, gasoline.
Solid. Usually carbolite parts can be recognized by a shiny surface and hardness, such plastic does not scratch the nail and does not even cling. Large flat parts almost do not bend, and if you exceed the effort with the sound of "crunch" break.
Well processed.Unlike many other plastics, it is well polished. If you try to grind, for example, polypropylene, then quickly from heating will begin to form a "beard" of plastic. Carbolite is perfectly polished and often you can see traces of grinding along the perimeter of the part - removing the burr.
Great appearance. The ability to form a solid glossy surface is especially noticeable in the appearance of retro equipment. Even in the store, on the shelf, the handles for carbolite resistors look more solid than those of thermoplastics.
High cost. The peculiarity of production in the form of pressing from powder determines a rather high cost of products due to the low speed of the process and the presence of manual labor. Making parts from thermoplastics is sometimes much cheaper.
Fragility. The reverse side of hardness, cracks in blows, do not make a
flexible hose, bellows, etc. out of it.
Practically not recyclable. There are ways, but they are not
widespread.
Limited color range. Phenol-formaldehyde resin itself is brown in color, making it difficult to obtain products of light colors. Melamine-formaldehyde resins, of which white products are made, are deprived of this disadvantage. Wonderful movie 40s, in which you can see the production of phenol-formaldehyde resin, molding parts by pressing, obtaining getinaksa, textolite, galalit and much more.
Getinaks is a laminate produced by pressing paper impregnated with
phenolic or epoxy resin. In English literature has the name FR-2. (FR - Flame Resistant is fire resistant) (FR-1, FR-2, FR-3 are all getinaxes , the difference is only in the binder material) We have GOST 2718-74 for getinax. It has low strength, but at the same time quite a low price. It is an insulating material, products from getinaks can be produced by stamping, therefore panels with lamellae, inserts, insulating washers, contact holders are sometimes made of getinaks.
Material cheap single-sided printed circuit boards. In tasks where high reliability is not required and it is possible to get along with one conductive layer, printed circuit boards are made of getinkix. In cheap electronic Chinese toys most often getinaksovy payments. Getinaks are not strong enough to create reliable vias, so double-sided and multi-layered printed circuit boards are not made of Getinaks.

Various products from getinaksa. The plate was intentionally broken to show a characteristic fracture pattern. Getinak bar slightly swelled to the right - the result of splitting layers when cutting.
Laminated getinaks (sloplast, laminated plastic) - getinaks with glued decorative film - material for interior decoration of buses, train cars, table tops. Durable wear-resistant slow-burning material.
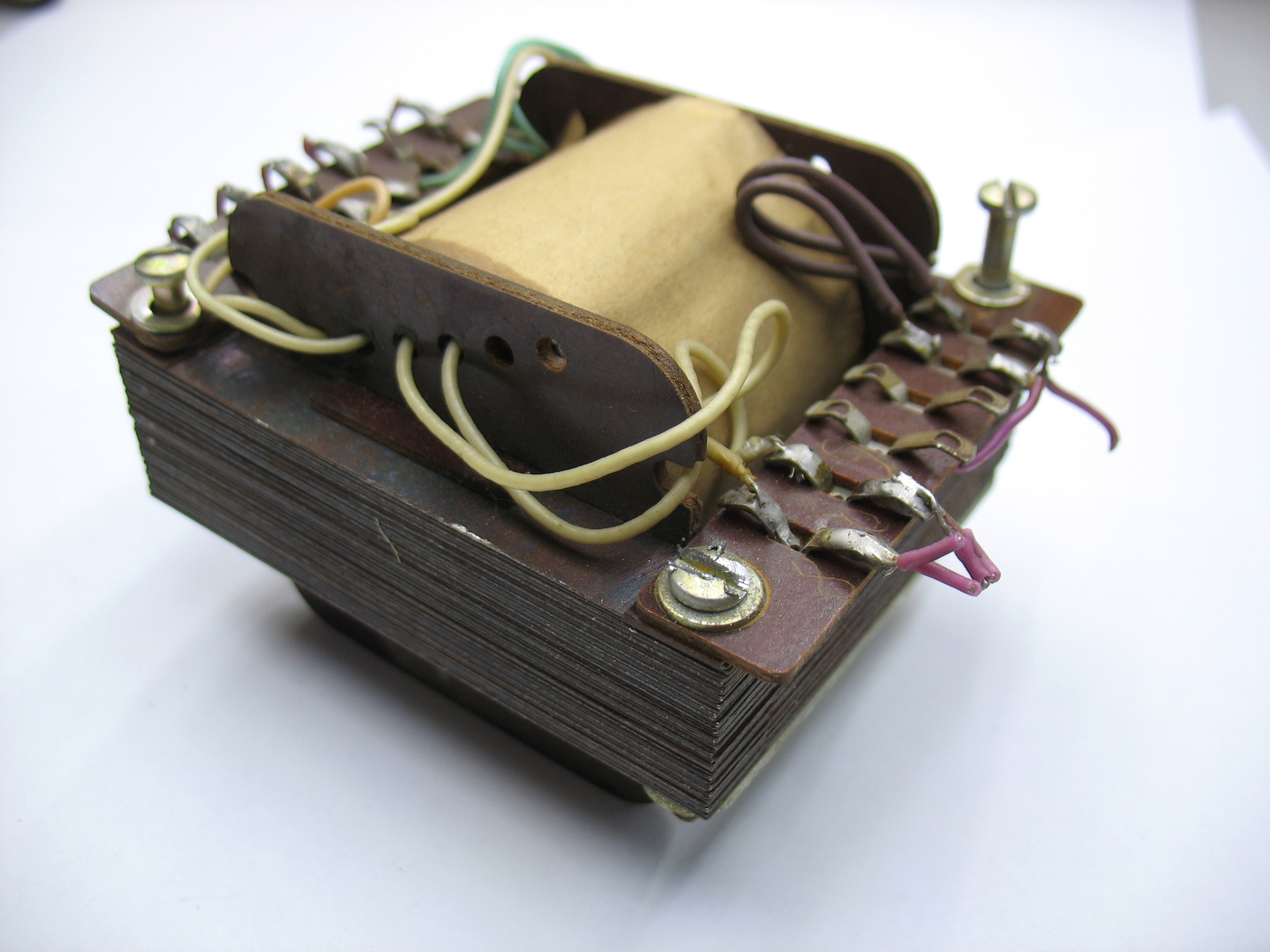
Transformer winding lamellas are made of getinax, insulating lamellas from the core lining, winding mandrel sidewalls are getinax.
The material is fragile and prone to crack when machining; special care is required when machining with a large tooth saw. Due to its low strength, it is of little use as a structural material.
Sold by many companies specializing in electrical materials.
Google on "Getinaks GOST2718-74".
Textolite is a whole class of composite materials, consisting of a pressed fabric with a binder. For example, cotton fabric impregnated with phenol-formaldehyde resin. It has a characteristic appearance - on the planes and sections, weaving of the fabric is visible. Usually brown and dark brown. Foreign brands are known under the trademarks Novotext, Turbax, Resitex, Cerolon, Textolit, Micarta. The material has been known since the 30s of the 20th century.

Textolite of various forms - plates, rods. The location of the fabric in the material varies - at the bars the fabric is wound and not laid in layers.
As a construction material. Textolite is durable and does not conduct current, therefore it is used as a material for gaskets, washers, partitions, inserts, gears, etc. When heated, it does not crawl, it distinguishes it from thermoplastic materials.
Ornamental material. From the PCB is often made the handles of knives, tools and tooling in small workshops. Textolite is well processed, it does not absorb water, it is resistant to the effects of fuels and lubricants.
Depending on the fabric used in the production, the observed texture may vary.

Textolite from fabrics with a different step of weaving. Textolite can always be recognized by its characteristic texture and appearance.
The material is available for sale in Russia, but gradually replaced by other materials.
1 : Conductors: Silver, Copper, Aluminum.
2 : Conductors: Iron, Gold, Nickel, Tungsten, Mercury.
3 : Conductors: Carbon, nichrome, thermostable alloys, solders, transparent conductors.
4 : Inorganic dielectrics: Porcelain, glass, mica, ceramics, asbestos, gas and water.
5 : Organic semi-synthetic dielectrics: Paper, click, paraffin, oil and wood.
6 : Synthetic dielectrics based on phenol-formaldehyde resins: carbolite (bakelite), getinax, textolite.
7 : Dielectrics: Glass fiber (FR-4), varnished cloth, rubber and ebonite.
8 : Plastics: polyethylene, polypropylene and polystyrene.
9: Plastics: polytetrafluoroethylene, polyvinyl chloride, polyethylene terephthalate and silicones.
10 : Plastics: polyamides, polyimides, polymethyl methacrylate and polycarbonate. History of use of plastics.
11 : Insulating tapes and tubes.
12 : Final

Welcome to CAT (TRAFFIC)
Available natural materials were widely used, but with the development of technology it became increasingly clear that natural materials are sometimes complete shit. There is a wide range of properties, susceptibility to rotting, difficulties in extraction - therefore, searches for artificial substitutes have been and are being conducted all the time. The appearance of synthetic materials is not only a technical revolution, but also an economic, political one. You no longer need colonies to cover your rubber needs. Equipping your soldier has become easier several times. In this section - the materials obtained "from scratch", and not an attempt to improve the natural, as in the previous section.
Many of these materials are polymers - materials with long molecules, consisting of simple bricks of the same type - monomers. Polymers can be divided into two large groups according to their behavior during heating; these are thermoplastics and thermoset plastics. Thermoplastics melt when heated, thermoset plastics decompose when heated.
Accordingly, a mountain of old plastic toys from thermoplastics can be melted down into a new product, and a mountain of old products from thermoset plastics cannot be recycled this way.
The polymer may consist of pure monomer, and may also contain a co-polymer, which is embedded in the structure of the molecule. For example, there are two monomers: A and B. A polymer molecule from pure A will look like this:
...- АА А А А А А А А А А А А А А А А А А…
A polymer molecule from copolymers A and B may look like this:
...- A-B-A-B-A-B-A-B-A-B-A-B- ...
Or even this way:
...- A- A-B-B-A-B-B-B-A-A-B-B- ... The
introduction of the copolymer allows you to change the properties of the plastic. An example is polystyrene and ABS plastic. Polystyrene is a transparent fragile plastic, the introduction of an acrylonitrile copolymer and the introduction of an additive from polybutadiene results in a shock-resistant plastic.
Sometimes, polymer stereoregularity may additionally be indicated. Suppose we have a monomer -G-, which can get up into the polymer chain "upside down" -L-. A polymer in which the chain of monomers are haphazardly called atactic:
...- L-G-L-G-LLL-G-G-L-G- ...
If in a polymer all unsymmetrical units look in one direction, such a polymer called isotactic:
...- LLLLLLLLLLLL- ...
If they alternate in a polymer, then such a polymer is called syndiotactic:
...- L-G-L-G-L-G-L-G-L-G-L-G- ...
Usually, stereoregularity affects the material properties of the material insignificantly, so it is not indicated.
General properties of polymers
Polymers, due to their structure of long molecules, have some common
properties that should be considered more closely.
1. Polymers do not have a clear phase transition temperature, such as metals. They are like caramel, soften with increasing temperature, turning into a viscous liquid. Therefore, for polymers, the “melting point” is the temperature at which the viscosity of the polymer already allows it to flow, but this does not mean that it is solid up to this temperature.
Glass transition temperature- is the temperature below which the polymer from a highly elastic state goes into a glassy state, with increasing hardness and brittleness. Imagine chewing marmalade - at room temperature, it is in a highly elastic state. If it is cooled below the glass transition temperature in the freezer, the marmalade can be broken, and the fragments will be as if from glass.
The maximum working temperature is the temperature at which the polymer can work for a long time, without significant changes in its properties. Often, as the temperature rises, the creep of the polymer increases, and therefore, at the maximum operating temperature, the strength properties decrease.
The indicated temperatures may differ in the determination even for the same sample, with different determination methods.
2. Polymers are subject to aging and degradation. Factors that accelerate the aging process of the polymer are radiation, ultraviolet radiation, high temperature, aggressive environment. Different polymers in different degrees of aging, in addition, various additives can reduce the rate of destruction of the polymer. Thus, a nylon tie on a silicone hose with hot water for a couple of years will lose elasticity and become brittle, while the silicone hose will still be soft and flexible.
Only a very small amount of plastics tolerate long-term heating above 100 ° C - fluoroplastic-4, Kapton, peek, silicones. In all other cases, the higher the operating temperature, the faster the aging and destruction processes in the polymer.
3. Polymers are permeable to gases and some solvents. Gas molecules are very small (the smaller the atomic mass, the smaller the size of the atom, the nastiest hydrogen in this regard, it squeezes even through the metals.) Therefore they can gradually penetrate the extensive molecular network of plastic. To prevent this process, the polymer surface is coated with a metal layer. Pay attention to this when opening the food packaging. Metallization in the package serves this purpose - do not miss the oxygen to the product. Metal-plastic pipes contain a layer of aluminum with the same purpose - to prevent the penetration of oxygen into the coolant, it causes corrosion.
Materials based on phenol-formaldehyde resins
Phenol-formaldehyde resins, as is easy to understand from the name, are products of phenol and formaldehyde polycondensation. Polymer molecules form a branched three-dimensional structure, which determines the mechanical properties - hardness.
Below we consider only phenol-formaldehyde plastics - phenoplasts . Urea-formaldehyde, melamine-formaldehyde plastics - aminos , we will not consider, their basic properties are identical, the processing methods are the same, the difference is only in strength, color.

The chemical structure of bakelite (piece for example) Polymers with such an extensive disordered structure are usually hard and brittle. The author of the picture is Dirk Hünniger, taken from Wikipedia.
Leo Bakeland, the American chemist from Belgium, opened the polycondensation process.
origin. He called the new material obtained during the curing of the resin - Bakelite.
In the USSR, a similar material was called "carbolite" - from carbolic acid, the
old name for phenol.
Examples of the use of phenol-formaldehyde resins:
- As independent material in the pure state as glues, varnishes.
- With powder fillers (giving strength or diluting material
- just to save) and without - carbolite / bakelite
- With fiberglass filling in a chaotic manner - fiber, for example press material AG-4V
- With a filling of layers of cotton fabric - Textolites
- With fiberglass filling - Fiberglass
- With filling from layers of glued paper - Getinaks
Carbolite (bakelite)
It is a solid heat-resistant plastic. If you take any device
assembled before 1950, then almost all plastic parts in it are carbolite.

Various products from carbolite - box, socket. Plug, voltmeter case, jacks, knobs adjustment.
Products are obtained both by pouring into molds and (more often) by pressing the resin powder with a filler into metal molds with heating. When heated, the polymerization process, which has already begun partly during the production of the powder, ends, but since the powder is squeezed under pressure at the moment in the mold, the appearance of the final product repeats the shape. A serious disadvantage of this method is that it takes time, which the product must spend in the form in order to gain strength enough to open the form without breaking, therefore in many tasks bakelite is displaced by thermoplastic materials, the molding machine can produce products of a given shape much faster.
A little about the process will tell this American advertising video of the last century, appreciate the enthusiasm with which they talk about the new material.

The body of the meter is made of carbolite.
Today products made of carbolite are mass produced, but it is not as popular as before, although there are tasks where it is difficult to replace it with something.
Buns
Heat-resistant plastic. It can work for a long time at a temperature of up to + 150 ° C. It is a thermoset - it does not melt, but is destroyed by heat. So carbolite cartridge for incandescent bulbs when overheating crumbles, and does not flow to your head.
Resistant to solvents, fuels and lubricants ( fuel and lubricants). Carbolite parts easily work near the car engine, in conditions of heating, contact with oil, gasoline.
Solid. Usually carbolite parts can be recognized by a shiny surface and hardness, such plastic does not scratch the nail and does not even cling. Large flat parts almost do not bend, and if you exceed the effort with the sound of "crunch" break.
Well processed.Unlike many other plastics, it is well polished. If you try to grind, for example, polypropylene, then quickly from heating will begin to form a "beard" of plastic. Carbolite is perfectly polished and often you can see traces of grinding along the perimeter of the part - removing the burr.
Great appearance. The ability to form a solid glossy surface is especially noticeable in the appearance of retro equipment. Even in the store, on the shelf, the handles for carbolite resistors look more solid than those of thermoplastics.
disadvantages
High cost. The peculiarity of production in the form of pressing from powder determines a rather high cost of products due to the low speed of the process and the presence of manual labor. Making parts from thermoplastics is sometimes much cheaper.
Fragility. The reverse side of hardness, cracks in blows, do not make a
flexible hose, bellows, etc. out of it.
Practically not recyclable. There are ways, but they are not
widespread.
Limited color range. Phenol-formaldehyde resin itself is brown in color, making it difficult to obtain products of light colors. Melamine-formaldehyde resins, of which white products are made, are deprived of this disadvantage. Wonderful movie 40s, in which you can see the production of phenol-formaldehyde resin, molding parts by pressing, obtaining getinaksa, textolite, galalit and much more.
Getinax
Getinaks is a laminate produced by pressing paper impregnated with
phenolic or epoxy resin. In English literature has the name FR-2. (FR - Flame Resistant is fire resistant) (FR-1, FR-2, FR-3 are all getinaxes , the difference is only in the binder material) We have GOST 2718-74 for getinax. It has low strength, but at the same time quite a low price. It is an insulating material, products from getinaks can be produced by stamping, therefore panels with lamellae, inserts, insulating washers, contact holders are sometimes made of getinaks.
Application examples
Material cheap single-sided printed circuit boards. In tasks where high reliability is not required and it is possible to get along with one conductive layer, printed circuit boards are made of getinkix. In cheap electronic Chinese toys most often getinaksovy payments. Getinaks are not strong enough to create reliable vias, so double-sided and multi-layered printed circuit boards are not made of Getinaks.

Various products from getinaksa. The plate was intentionally broken to show a characteristic fracture pattern. Getinak bar slightly swelled to the right - the result of splitting layers when cutting.
Laminated getinaks (sloplast, laminated plastic) - getinaks with glued decorative film - material for interior decoration of buses, train cars, table tops. Durable wear-resistant slow-burning material.

Transformer winding lamellas are made of getinax, insulating lamellas from the core lining, winding mandrel sidewalls are getinax.
Note
The material is fragile and prone to crack when machining; special care is required when machining with a large tooth saw. Due to its low strength, it is of little use as a structural material.
Sources
Sold by many companies specializing in electrical materials.
Google on "Getinaks GOST2718-74".
Textolite
Textolite is a whole class of composite materials, consisting of a pressed fabric with a binder. For example, cotton fabric impregnated with phenol-formaldehyde resin. It has a characteristic appearance - on the planes and sections, weaving of the fabric is visible. Usually brown and dark brown. Foreign brands are known under the trademarks Novotext, Turbax, Resitex, Cerolon, Textolit, Micarta. The material has been known since the 30s of the 20th century.

Textolite of various forms - plates, rods. The location of the fabric in the material varies - at the bars the fabric is wound and not laid in layers.
Application examples
As a construction material. Textolite is durable and does not conduct current, therefore it is used as a material for gaskets, washers, partitions, inserts, gears, etc. When heated, it does not crawl, it distinguishes it from thermoplastic materials.
Ornamental material. From the PCB is often made the handles of knives, tools and tooling in small workshops. Textolite is well processed, it does not absorb water, it is resistant to the effects of fuels and lubricants.
Depending on the fabric used in the production, the observed texture may vary.

Textolite from fabrics with a different step of weaving. Textolite can always be recognized by its characteristic texture and appearance.
The material is available for sale in Russia, but gradually replaced by other materials.
Links to parts of the manual:
1 : Conductors: Silver, Copper, Aluminum.
2 : Conductors: Iron, Gold, Nickel, Tungsten, Mercury.
3 : Conductors: Carbon, nichrome, thermostable alloys, solders, transparent conductors.
4 : Inorganic dielectrics: Porcelain, glass, mica, ceramics, asbestos, gas and water.
5 : Organic semi-synthetic dielectrics: Paper, click, paraffin, oil and wood.
6 : Synthetic dielectrics based on phenol-formaldehyde resins: carbolite (bakelite), getinax, textolite.
7 : Dielectrics: Glass fiber (FR-4), varnished cloth, rubber and ebonite.
8 : Plastics: polyethylene, polypropylene and polystyrene.
9: Plastics: polytetrafluoroethylene, polyvinyl chloride, polyethylene terephthalate and silicones.
10 : Plastics: polyamides, polyimides, polymethyl methacrylate and polycarbonate. History of use of plastics.
11 : Insulating tapes and tubes.
12 : Final
