Scientists have proposed a method for creating flexible supercapacitors capable of fully charging a smartphone in seconds.
 A team of scientists from the Center for Nanotechnology at the University of Central Florida (UCF) has developed a new method for creating flexible supercapacitors . They accumulate more energy and withstand over 30,000 charge cycles without damage. The new method of creating nanocapacitors can be a revolutionary technology in the production of smartphones and electric vehicles.
A team of scientists from the Center for Nanotechnology at the University of Central Florida (UCF) has developed a new method for creating flexible supercapacitors . They accumulate more energy and withstand over 30,000 charge cycles without damage. The new method of creating nanocapacitors can be a revolutionary technology in the production of smartphones and electric vehicles. The creators are sure: if you replace the usual batteries with new nanocapacitors, then any smartphone will fully charge in a few seconds. The owner may not think every few hours about where to charge his smartphone: the device will not be discharged within a week.
Every smartphone owner is faced with a still unsolvable problem: approximately 18 months after the purchase, the average battery keeps the charge less and less time and then finally degrades. To solve it, scientists are exploring the possibilities of nanomaterials to improve supercapacitors. In the future, they can support or even replace batteries in electronic devices. It is quite difficult to achieve this: in order for an ionistor to conduct as much energy as a lithium-ion battery, it must significantly exceed the usual battery in size.
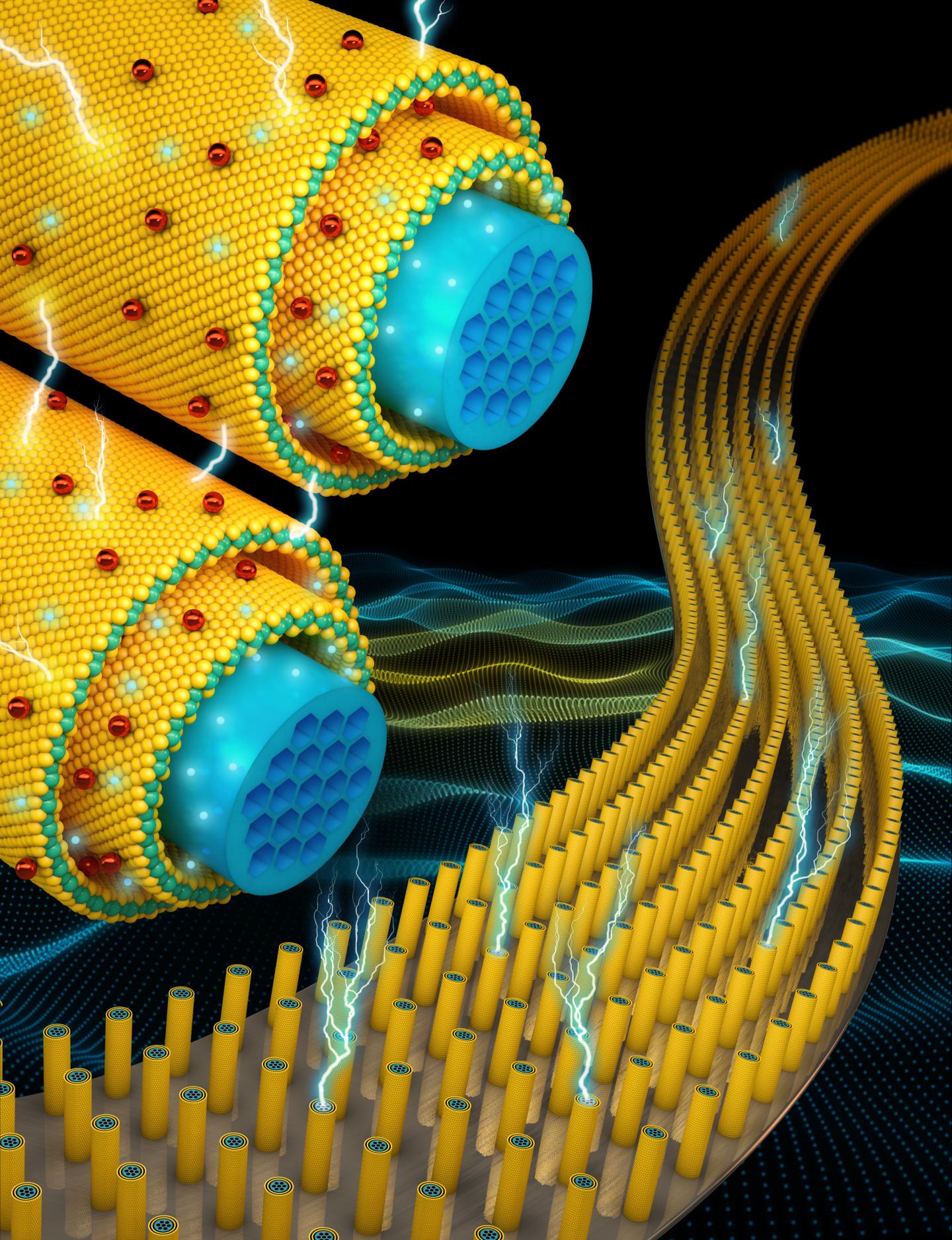
A team from UCF experimented with recently discovered two-dimensional materials several atoms thick — thin films of transition metal dichalcogenides ( TMDs ). Other scientists tried to work withgraphene and other two-dimensional materials , but it cannot be said that these attempts were quite successful.

Two-dimensional transitional materials dichalcogenides are a promising material for capacitive supercapacitors due to their layered structure and large surface area. Previous experiences integrating TMDs with other nanomaterials have improved the electrochemical characteristics of the former. However, such hybrids could not withstand a sufficient number of recharge cycles. This was due to the violation of the structural integrity of materials in the joints with each other and chaotic assembly.
All scientists, who somehow tried to improve existing technologies, wondered: "How to combine two-dimensional materials with existing systems?". Then a team from UCF developed a simple chemical synthesis approach, with which it is possible to successfully integrate existing materials with two-dimensional metal dichalcogenides. This was stated by lead author Eric Jung.
The Young team developed supercapacitors consisting of millions of nanometer-long wires coated with transition metal dichalcogenides. A nucleus with high electrical conductivity provides fast electron transfer for fast charging and discharging. Uniform shell of two-dimensional materials has a high energy intensity and power density.
Scientists are confident that two-dimensional materials open up broad prospects for energy storage elements. But until the researchers at UCF came up with a way to combine the materials, it was not possible to realize this potential. “Our materials, designed for small electronic devices, have surpassed conventional technologies all over the world in terms of energy density, power density and cyclic stability,” said Dr. Nitin Choudhary, who conducted a series of studies.
Cyclic stability determines how many times a battery can be charged, discharged, and recharged before it starts to degrade. Modern lithium-ion batteries can be charged about 1.5 thousand times without serious failures. A newly developed prototype supercapacitor can withstand several thousand such cycles. The two-dimensional shell ionistor did not degrade even after it was recharged 30 thousand times. Now Jung and his team are working to patent a new method.
Nanocapacitors can be used in smartphones, electric cars, and in fact - in any electronic devices. They could help manufacturers benefit from sudden changes in power and speed. Since ionistors are quite flexible, they are suitable for wearable electronics and technology.
Despite all the advantages of the new supercapacitor, the development is not yet ready for commercialization. Nevertheless, this study may be another serious impetus for the development of high technology.
The scientific work was published in the journal ACS Nano October 12, 2016
DOI: 10.1021 / acsnano.6b06111
