How genomic editing will change agriculture. If we allow ...
 In the light of the recently adopted ban on GMOs ( post ), the topic of genetic engineering is widely known. I offer you a translation of an excellent article that describes the history of biotechnology in agriculture and raises the question of the applicability of the term GMO to the products of the new generation technician. This gives hope for the use of new organisms without the hell of bureaucracy and panic among the population.
In the light of the recently adopted ban on GMOs ( post ), the topic of genetic engineering is widely known. I offer you a translation of an excellent article that describes the history of biotechnology in agriculture and raises the question of the applicability of the term GMO to the products of the new generation technician. This gives hope for the use of new organisms without the hell of bureaucracy and panic among the population.Technology
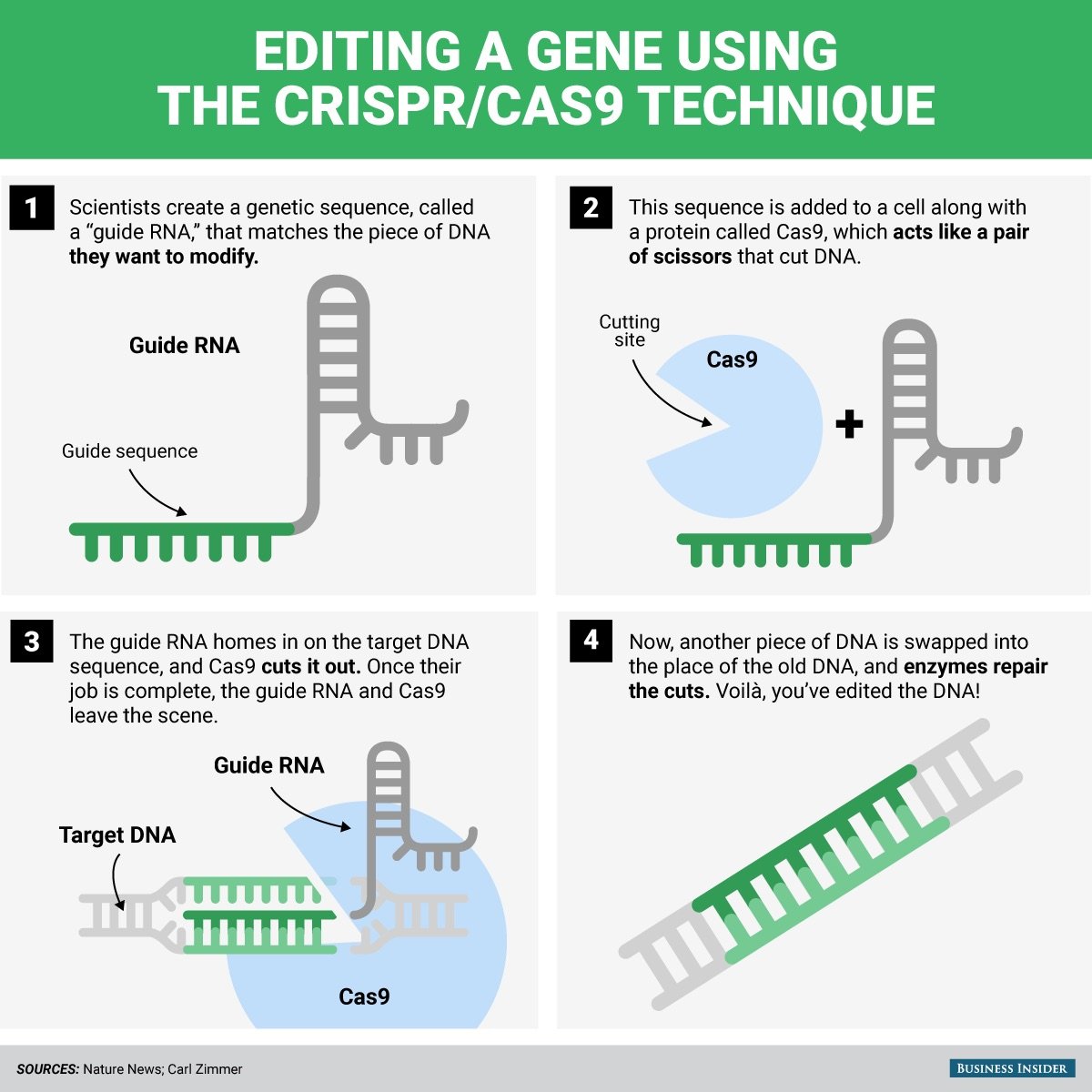
Genomic editing is essentially a simple idea — meaningfully making a change in a particular area of the body’s genome, usually in a gene. This mutation (a change in the DNA sequence of a gene) leads to a change in the protein encoded by this gene, which translates into a physically noticeable feature in the body. Over the past 4 years, genomic editing has become famous and famous; almost all the media from the Guardian to the KP mentioned this, there were several TED lectures , articles on technical sites and, perhaps more important, on Buzzfeed .
In the scientific community, genomic editing has become a magnet for grants, publications and citations - the holy trinity of modern science. The reason for such significant interest lies in the recent discovery and improvement of the CRISPR / Cas9 technology, a simple tool that made genomic editing available to most biological laboratories around the world. Over the 4 years since the debut, CRISPR has been used to edit human cells, cereals, insects (for example, butterflies), yeast, and many others.

Scientists from Cornell University recently edited the genome of Vanessa's butterflies to search for the genetic basis in the formation of "eyes" on the wings.
Before CRISPR, it was almost impossible to create a mutation only in the target gene and do so with relative ease. It is quite difficult for a layman to explain how advanced CRISPR is compared to earlier genome editing techniques; This can be compared with the technological leap from the ancestors of engines to the internal combustion engine. Of course, this whole conversation about target mutations raises the question: “Why would we do this at all?”.
Since the moment the human genome became known, biologists have been able to learn a lot about the genetics of diseases. For example, we know that Huntington's disease (an incurable genetic disorder) is caused by too many CAG bases in the HTT gene sequence. Or that sickle cell anemia is caused by a single substitution of A-> T in the hemoglobin gene (a protein that makes blood red and binds oxygen). However, nothing special can be done with this, except for diagnostics and genetic counseling. Imagine the frustration that a doctor must feel, knowing the cause of the illness and not being able to do anything. And with CRISPR, we may be able to understand how to properly edit these genes and fix the mutations that caused the diseases.
As in the case of any new bioengineering techniques, CRISPR is primarily trying to apply for medicine. But, although these capabilities are rapidly being developed in laboratories around the world, it is still far from real use. But the industry, which can be almost immediately changed by CRISPR and editing, in principle, is agrotechnology.
Natural variability
The history of genomic editing in agriculture should begin with a story about natural genetic variability and its limits. Most plants have a very significant percentage of total DNA; This fact seems obvious if you look at how many basic physiological functions (from photosynthesis to reproduction) combine different types of plants (and, generally speaking, most living things have quite a lot of common DNA). However, the same genes, even within the same species, may differ; this difference can be as small as the replacement of one nucleotide for another, and as large as the loss of a whole piece of a gene. Genes can be represented as one or more copies in the genome or absent altogether (which is also essentially a mutation, only larger). What I'm trying to convey here is the fact that although so many genes are the same within species and between species, most of them still have small differences or mutations. These mutations are mainly the result of evolution and these different versions of the same gene are called alleles . This diversity of alleles can change the function of genes in a variety of ways - from turning it off completely to changing what this gene actually does.
In this vein, the whole history of agriculture is an attempt to choose the most suitable and discard less useful alleles for breeding work.
Usually, farmers and breeders make it painful and not the most effective way - they study large plant populations and cross them with each other until they get a plant that has the right combination of “useful” alleles of different genes. And naturally, what is good for one population, may be bad for another, which makes the availability of genetic diversity very important.
We save diversity

World Seed Storage on about. Svalbard, one of the largest in the world (Photo: Mari Tefre / Svalbard Globale frøhvelv) The
existence of a large variety and variability of alleles is fundamental to the crossing process. In fact, without a large set of different variants of genetic material, crossing would not make sense at all (it would have the same comment.). The good news is that farmers and scientists understand the importance of biodiversity, and in about 1,300 special repositories there are seeds of approximately 6 million plant varieties (species and species). With all this, the preservation of the variability is not the same as its use. Breeders often use “wild” relatives of cultivated plants and old crossbreeding results as a source of new alleles, while a large amount of material in the seed stores remains undescribed and unused.
New DivSeek Initiative(led by scientists and biodiversity experts from 65 organizations worldwide) addresses this issue. DivSeek assumes a description of the genome and phenotype (of how this genotype is implemented in a particular plant) of samples presented in repositories and the presentation of the data obtained in open access. This is a very ambitious project, even the choice of which of the millions of samples to test is already a difficult question. By-products of this task can be 1) reducing the price of DNA sequencing 2) automated pipelines for studying phenotypes with high throughput and 3) dissemination and exchange of information between farmers. This recent initiative is designed for a long time and began work quite recently, but its success may mean a new era in the study of the diversity of cultivated plants.
Creating diversity
Eisenhower propaganda poster Atoms for Peace. According to the National archives It is a
little known fact that a significant number of modern popular agricultural plant species were obtained as a result of the mutagenesis research program in the early and mid-20th century; This is partly a by-product of the development of nuclear technology and the US Atoms for Peace state program .
Classical mutagenesis to create variability in plants is to create mutations in the seeds either by X-ray / gamma radiation or by using chemical mutagens. These mutagens cause damage to the plant's DNA and the restoration of these damage leads to the creation of new, mutant alleles. The obtained alleles can be both unique and in fact already existing in natural plants (for example, not in those where mutagenesis is done). Mutation using this technology is inaccurate, several million mutational events occur in the genome, with only a fewpart of them is needed for crossing. Therefore, the primary products of mutagenesis must undergo a selection and a series of crosses in order to select potentially useful alleles and introduce them into the existing species of the cultivated plant; This process is long and can take decades. Mutational selection is therefore expensive, and takes a lot of time, but it still creates additional alleles and, accordingly, diversity. Some of these products of such mutation breeding are still widely used, for example, dwarf wheat (dwarf wheat), famous for the Green Revolution, dwarf rice in California, resistant to coconut viruses in Ghana, and cereals that go through malting better in Europe.
Genomic editing is another form of mutagenesis. The important difference here is that the old methodologies rely on random events, while genomic editing is accurate and focused, which leads to a sharp reduction in time from mutation to the sowing of experimental plants.
New agriculture
Plant breeding is even now described in textbooks as “art and science” . In many ways, because it depends on the skills of the breeding specialist to select various properties of plants - the so-called breeder eye. Traditional crossbreeding also requires a huge amount of time and resources, as it uses reboring approaches to finding new plant properties and creating species diversity.
In many ways, everything happens exactly because we have gaps in the biology of certain properties, these gaps will be filled by the next generations of biologists, but, despite the lack of such knowledge in the past, this did not prevent the breeders from doing amazing work on creating various plants and increasing yields.
The history of plant breeding is a development from the “black box” to a more complete understanding of what the plant does and how best to use their unique properties. Our ancestors realized that planting seeds of plants that had more fruit or fewer diseases, gave them a greater harvest the next season, but they remained blind in questions of the biology of reproduction. Much later, in the 17th century, we understood more about how plants are reproduced and began to make artificial crosses. Soon after, Darwin and Mendel came to us who gave us the ideas of natural selection and the laws of genetics, and all this for 50 years! And now, with the recent proliferation of -omics technologies, we can read the DNA of plants, study how eachThe gene responds to different environmental conditions and predicts how efficiently a plant can produce chemicals for the sake of which we eat them. This knowledge becomes even more useful in the case of genomic editing.
Once a breeder or scientist has found a useful allele, using genomic editing, they will be able to transfer it to another variety or even a plant species practically immediately, without the need for a series of generations.
In the future, genomic editing may change the process of obtaining new properties (alleles) as such. CRISPR-based genomic editing can be used to simultaneously edit each gene in a plant's genome (or each gene of a particular type — for example, the R-genes that are responsible for disease resistance), thus creating a lot of information and potentially opening useful alleles that can be inserted back into already used plant varieties. The real top of genome editing with CRISPR is the ability to create different alleles separated from sexual reproduction.
Genomic editing can, it seems to me, provide a plug-and-play model for plant breeding.
The selection pipeline of the future will be in my estimation similar to modern conveyors. Using data from 1000 scientific articles and initiatives such as DivSeek, researchers will test various combinations of alleles in model plant varieties by directly editing their genome, possibly with the help of experts in predictive analysis and mathematical modeling. After selecting alleles based on these results, scientists will be able to use these changes on a large number of non-model plant varieties, carry out tests in the field and start producing seeds of new varieties. Although there are a lot of factors influencing the process, the greatest effect in agriculture will be provided by a decrease in the number of required generations for the new variety test. In other words, faster product creation.
Students studying plant immunity are familiar with the zigzag model of the coevolution of plants and pests. This model describes the arms race between the plant and the pathogens that attack it, and many of them quickly acquire evolutionary changes than the plant. The task of modern agriculture is like that. Industries need to feed an increasing population of people, cope with the effects of climate change (increasing number of extreme natural phenomena in the short term and global climate change in more distant prospects) besides coping with rapidly changing pests and all this with the requirement for sustainability of the resulting system.

When will there be a society of universal affluence?
Agriculture, of course, has encountered similar problems in the past, for example, the Green Revolution, which is particularly well known, has disproved the predictions of Paul Ehrlich. This revolution became possible only because it was headed by the Nobel Peace Prize winner Norman Borlaug , who presented new plant varieties and mechanization tools for farmers.
Now we are faced with the same obstacles, but with even bigger ones and staying in place means moving backwards.
Much of what has been discussed has been discussed before, at the time of the emergence of new agricultural technologies: from hybridization of cereals and genetic engineering to selection using markers. Some of these technologies have been adopted; but genetic engineering remains monopolized by several large companies, rejected by many nations and is considered only in the distant future. What fate awaits genomic editing?
Wait, is it GMO?
This question is constantly asked when I talk about genomic editing, and from the point of view of government regulation, this is the question that fully determines the fate of this technology in agriculture (I don’t want to enter into a debate on the regulation of GMOs in this article, and I assume that the situation not yet changed in the near future, especially in Europe).
Answering the original question: I don’t think so - for the simple reason that you can’t tell the difference between the edited plant and the natural variant found in nature. The result of genomic editing usually does not contain any transgenes (those genes that are taken from another organism are not found in nature in the modified one) and, in all likelihood, andthere will be no traces of the method by which the grade is editedplants. This poses an amazing problem for regulators and public groups that would like to consider editing as one of the forms of creating GMOs (that is, applying the same laws to it). How can you regulate this area if you don’t know to which plants (is it natural or created?) Does the law apply and which ones don’t? Of course, you can check breeding companies and laboratories or try to make the process of obtaining basic reagents very difficult (difficult job), but is this necessary for society, to follow private firms and non-business scientists? Organizations like Greenpeace or Friends of the Earth, like the “organic industry” want to regulate genomic editing products, but I have not seen a single intelligible proposal, how could this be organized.
And now at a more fundamental level, how exactly does genomic editing differ from random mutagenesis? Editing is performed using biochemical agents (RNA and proteins), which act more accurately than UV or chemical mutagens; the main thing is that the end product is the same - a plant with a new allele. Now you may want to consider random mutagenesis as a process of genetic modification. And you will be absolutely right, as there is a modification of the genetic material.plants. But it is important to understand that countries make exceptions for random mutagenesis for two reasons: a) this is the part of modern agriculture that cannot be left at all (including organic agriculture) and b) the result cannot be distinguished from natural variation either. Therefore, we are arguing about anything , trying to classify the result of one technology as GMO, and the second as non-GMO. It is obvious to scientists that there is no separation of GMO / non-GMO in nature.
Back to our question:
“Is it GMO?” - technically, yes (like many plants grown by “organic” farmers around the world).
“Is it important?” Nah.
Who will own these plants?
Another frequent question I get asked when I describe my current project to create a transgenic plant is “Will it be patented?”. Many criticisms are aimed at protecting intellectual property (plant varieties) such as Greenpeace: “Existing living organisms are plants and animals, as well as their genes, no one’s invention, and therefore they should never be patented or put under private control.” This statement implicitly implies that those are the cultures that we are currently using “nobody's development”. In response to this, I hope that I clearly showed how far agriculture depends on the skills and ingenuity of farmers, breeders and, yes, modern biotech companies. In the corn picture you can see how human ingenuity created a system that could feed more people every day.

On the left, teosinte is the genetic precursor of corn, and on the right is the familiar domesticated corn. (c) John Dobley
The right to intellectual property is extremely important in the development of modern technologies and legal circumstances will play a significant role in the application of genomic editing in agriculture.
Brief description of IP protection systems in the USA and Europe
The inventor of a new plant variety usually has two options for protection - plant variety protection (PVP) or a patent (usually a patent for use in the United States). Patents imply a high degree of scientific novelty and are mainly used by biotechnology corporations to protect seed material using a patent for a DNA sequence or a property that has been added to a plant. PVP is used by breeders using traditional methods; there are less stringent criteria for novelty, but a little less protection. For example, the patent does not allow farmers to use seeds for re-sowing or breeders to create new varieties based on the patented, while PVP allows it. In the United States, unlike many countries, patents on breeding varieties are allowed, as well as plant creation methods,
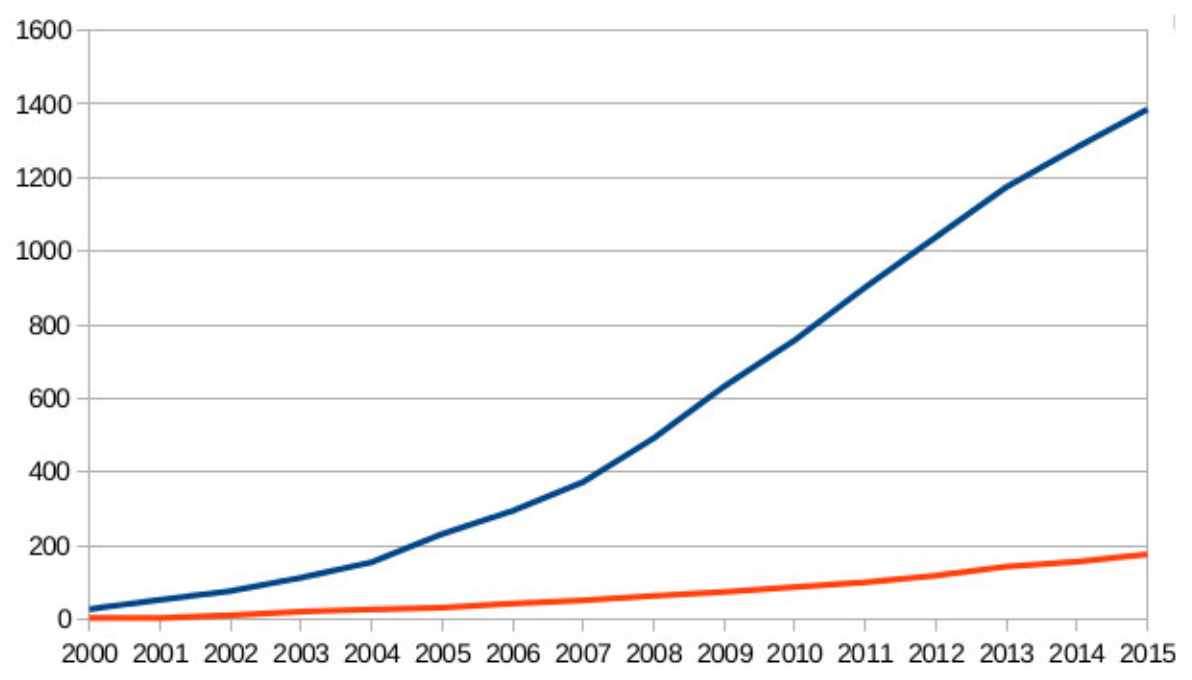
There is definitely a demand for patents! Requests - the blue line, received patents - red. According to NoPatentsOnSeeds
In Europe, breeders usually receive PVP because there patents are not granted for “essentially a biological selection process”. However, the situation is not so simple (see chart) and the recent decision of the European Patent Office (EPO) introduces the difference between the process of obtaining new varieties (they cannot be patented) and the results (but they seem to be as good as possible). For example, EPO issued a patent to the Israeli Ministry of Agriculture for drought tolerant tomato obtained by traditional breeding methods. It is worth waiting for the decision of the European Commission, which promised to deal with this situation, at the moment they are working on a legally binding interpretation, which may prohibit patents on the cultivated varieties.
Currently, in Europe, the results of genetic engineering can be patented, but the result of traditional selection is not. This situation is somewhat puzzling, because if regulators consider that genomic editing of a non-GMO technique, can it be made a patent or will you have to limit PVP? On the one hand, if we consider this a genetic modification (therefore patented), then how can the applicant prove that his body is unique (that is, there is no allele in nature that would coincide with the claimed organism)?
Another point is how patents affect the prevalence of certain technologies. Patents give the inventor a monopoly for a certain time (20 years in the US). This does not limit the ability to use genetically modified DNA in other plant varieties by other breeders unless another breeder buys a license for it. But if the “edited” plants meet the requirements for PVP, then the alleles of these plants can (and most likely will be) widely used by other breeders and farmers. Therefore, the question of whether the industry will require initial investment to launch projects with an edited genome remains in force.
Where will the money come from?
Virtually every agrobiotech company today invests in editing at least a few plants. For example, a small company from the USA Cibus is already planning to produce herbicide-resistant canola this year. Although it is this plant that was developed with the help of another, older technology, it can be said with confidence that new developments will be based on CRISPR technology. Currently CRISPR patents are with the Broad Institute MIT and the Harvard Institute, DuPont and several other organizations. As I understand it, DuPont and Caribou Biosciences have the strongest positions in agrobiotech (spin-offs from Berkeley). But on the other hand, technology is developing rapidly.and new methods are emerging from other universities and companies.
The key point here is that if patent protection extends only to the method of obtaining the product, but not to the product itself, then are companies ready to invest in editing. In the USA, where products can be patented, the situation is different, but for example, Monsanto gets ~ 40% of revenue not in the USA, then this is still an urgent issue even for America. For companies outside the US, all this is even more important. It may take a more intelligent protection of intellectual property to encourage innovation in this environment.
The first steps
Some moments in the article are somewhat speculative, but surprisingly most are not. The genome was edited using CRISPR in a wide variety of plants — rice and wheat , tomatoes, and lettuce . Edited plants are getting closer to entering the market: both Cibus and DuPont are already testing in the field.
With all this, plant specialists still need to further develop tools that will allow them to carry out development using the “plug and play” model, which was described above. The first stage of genomic editing is a sustainable genetic transformation.and the regeneration of the “stripped” plant cell (protoplast) and in my opinion not enough scientific projects are working on this basic task of cultivating plant tissues of various species. We also need to improve the systems for predicting the results of genetic interventions in a particular gene or genomic region; perhaps full-genome models could help with this. We need more productive phenotyping systems, for example, single-cell systems to test for immune responses. In this we must take an example from the biomedical community, where, for example, microfluidic systems for the cultivation of cell cultures, but for plants? The cost of sequencing the human genome has dropped to $ 1000 per genome and drops to $ 100 / genome.. Similar progress is needed for plants, perhaps even more, since their genomes are more complex than in humans.
It is absolutely clear that the wide distribution of genomic editing in agriculture depends on how this technology is regulated. As a supporter of this idea, I believe that the United States is on the right track with its systems of regulation and protection of intellectual property. Discussions in the European Commission are also very significant because of the important role of the EU in FAO and the World Intellectual Property Organization, let Europe produce not so much food.
Given the growth of the population and the dynamics of climate change, it is clear that the current technology will not feed the world. The most severe blow will be to the poorest southern countries, whose economies cannot subsidize agriculture in developed countries. It is possible that to create more sustainable agriculture it is worth being more open to new technologies and not concentrating on less efficient ones . Most likely it will not work to reduce the amount of food consumed, but you can increase the efficiency with which we produce it.
