New material for fuel cells allows you to create long-term "energy cells"
Lithium batteries are an excellent solution for storing energy generated by solar panels or other sources of green electricity. But they are discharged quickly enough, so this is a short-term solution - to save energy “for the future” will not work. In addition, very massive storage facilities are needed to store really large amounts of energy (one such was built by Elon Musk in Australia).
Experts have been looking for a suitable solution for many years, but so far nothing radical has been created. True, recently fuel cells that generate energy from, for example, hydrogen, have become more popular. The other day it became known about a new type of fuel cells that work in two directions at once - they can generate electricity from methane or hydrogen, or they can consume energy and produce methane or hydrogen.
The efficiency of the cell is quite high: if you spend a certain amount of energy on the production of methane or hydrogen, and then let everything go in the opposite direction, then you can get 75% of the electricity spent earlier. In principle, very good.
Limitations
Batteries, as mentioned above, are not too good for long-term supplies of electricity. Other disadvantages are the slow recharging speed plus the high cost. A good solution can be flowing batteries, which are used more and more.
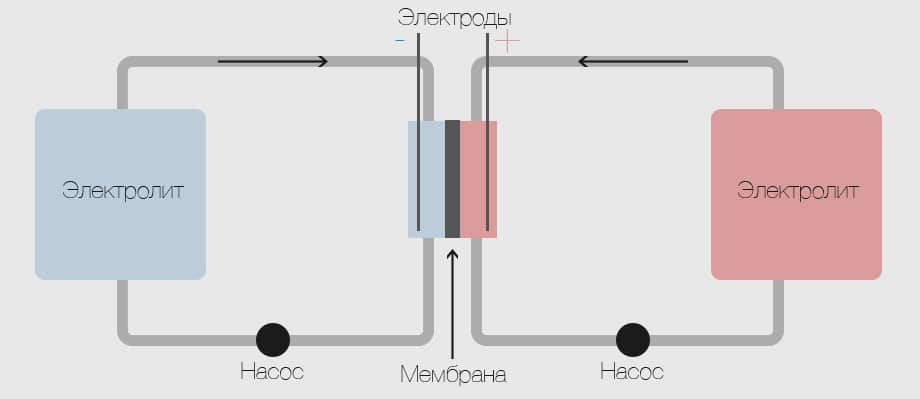
A flowing (redox) battery is an electrical energy storage device that is a cross between a conventional battery and a fuel cell. A liquid electrolyte, consisting of a solution of metal salts, is pumped through the core, which consists of a positive and negative electrode, separated by a membrane. The ion exchange arising between the cathode and anode leads to the generation of electricity.
But flowing batteries are not as effective as traditional batteries, but the electrolyte that is used in them is usually toxic or causes corrosion (and sometimes both).
An alternative to storing energy over time is to turn surplus electricity into fuel. But everything is not so simple here, the usual schemes for converting energy to fuel are energy-intensive enough, so the efficiency of the system will never be high. In addition, reaction catalysts are usually expensive.
A way to reduce costs is to use a reversible (reverse) fuel cell. In principle, they are not new. When working in the forward direction, fuel cells take hydrogen or methane as fuel and generate electricity. Working in the opposite direction, they generate fuel by consuming electricity.
Just reversible fuel cells are ideal for long-term energy storage, as well as for the production of methane or hydrogen where they are needed.
Why are they not yet used universally? Because in theory everything looks great, but in practice there are insurmountable difficulties. Firstly, many of these elements need a high temperature to work. Secondly, they produce a mixture of hydrogen and water, rather than pure hydrogen (in most cases). Thirdly, the cycle efficiency is very small. Fourth, the catalyst in most existing elements is rapidly destroyed.
Way out
It was proposed by researchers from the Colorado School of Mines. They studied the possibilities of reversible proton-ceramic electrochemical cells. When generating energy, they are very efficient, plus they do not need a very high temperature - there are enough sources of waste heat from industrial processes or traditional production of electricity.
Scientists have improved the technology by offering Ba / Ce / Zr / Y / Yb and Ba / Co / Zr / Y as the material for the electrodes. For their work, a temperature of 500 degrees Celsius is needed, which is not a problem, plus about 97% of the energy that was supplied to the system is involved in production. In this case, the cells operate on water or water and carbon dioxide. They produce hydrogen, in the first case, or methane, in the second.
The system efficiency is about 75%. Not as good as the batteries, but for most purposes this is enough. In this case, the electrodes are not destroyed. After 1200 hours of testing, it turned out that the material practically did not degrade.
True, another problem remains - the high cost of the starting materials that are used to create the electrodes. The same ytterbium costs about $ 14,000 per kilogram, so creating really large fuel cells can be quite expensive.
But, perhaps, the developers will be able to solve this problem - in any case, work in this direction is already underway.


