Notes phytochemist. Radio banana
- Tutorial
Every miracle must find its explanation, otherwise it is simply unbearable ...
C. Chapek
I practically do not touch in my articles of things that are universally described and easily accessible, for example, the macro-and microelement composition of fruits / vegetables. But for a banana decided to make an exception. Banana has a lot of potassium! Lift anyone in the middle of the night and ask what is useful in a banana - you will get the answer “potassium for the heart” (I exaggerate, but not far from the truth). And potassium, it is an element difficult, "with a hint of radioactive ...". In general, to find out whether the radioactivity from a banana is so great and whether it is so terrible - go under the cat.
ps note "on request ..."
Potassium refers to the so-called. nutrients , i.e. it is constantly present in a living organism and plays an important biological role. The human body contains about 0.35% potassium. 98% of this amount is in the cells, and the remaining 2% is extracellular fluid (including blood). Gradient concentrations supported by the so-called. "Na + / K +pump. The fact that there is an electrochemical potassium gradient between the intracellular and extracellular spaces is important for the functioning of the nerve function (cell membrane repolarization, for example). With hypokalemia (potassium deficiency), because of slower ventricular repolarization, the risk of heart rhythm disturbances, which can often lead to cardiac arrest, is increased. In general, it is clear that the body is very necessary. It comes in, in most cases (like other trace elements) with food.
Important! If necessary, clarify some data on certain trace elements / amino acids, etc., I use the database of the US Department of Agriculture (United States Department of Agriculture Agricultural Research Service, it is USDA) and I strongly advise you. Objective source, in my opinion, does not exist.
So, according to this database, in bananas about 358 mg of potassium per 100 g of product, only kiwi with its 522 mg of potassium has comparable "power" of the available tropical "guests". Everything else is quite rare (tamarind - 628 mg, avocado - 485 mg (not rare, it is often found in sushi), durian - 436 mg, guava - 417 mg, passion fruit - 348 mg). At the same time, compare with the relatives “at each exit from the metro” products: dill - 738 mg, spinach - 558 mg, parsley - 554 mg, cilantro - 521 mg, even sorrel is forest and that contains 390 mg per 100 grams of the product. There are also vegetables in Coy: Brussels sprouts - 389 mg, pumpkin - 340 mg, black currants - 322 mg. So before the next "find% nutrient% in 60 seconds on a shelf with subtropical fruits," take a look at the USDA base, maybe everything is already in a carrot or zucchini ...
In any vegetable / fruit / herbs besides potassium, there are its isotopes . Stable are 39 K (93.08% of the total mass), 40 K (0.01% of the total mass, half-life 1.248 * 10 9 years), 41 K (6.91% of the total mass). All the rest live from hours to nanoseconds and break up:
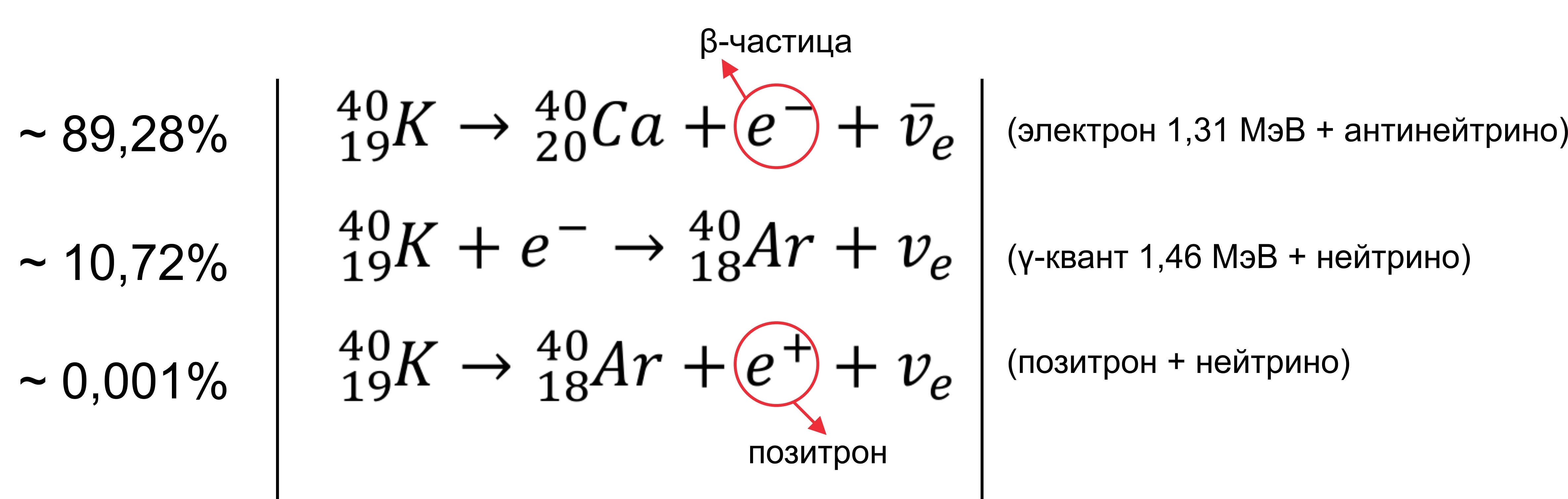
Our trace element (relative to others) is unusual in that it has an isotope of 40 K, which is a rare example of an isotope that undergoes both types of beta decay. Approximately 89.28% of the cases it decomposes to calcium-40 ( 40 Ca) from the emission of a beta particle (β - , electron) with a maximum energy of 1.31 MeV and antineutrinos. About 10.72% of the time, it decays into argon-40 ( 40 Ar) by capturing electrons with the emission of gamma radiation with an energy of 1.460 MeV and neutrinos. The radioactive decay of this particular isotope explains the high argon content (almost 1%) in the earth’s atmosphere, as well as its high content compared to 36 Ar. Very rarely (in 0.001% of cases) it breaks up to 40Ar, emitting a positron (β + ) and a neutrino. About the last reaction was written in the Habra article . Say banana source of antimatter.
Due to the facts, 40 K is the largest source of natural radioactivity in animals, including humans. In a gram of natural potassium, an average of 32 decays of potassium-40 occurs per second (32 Becquerel, or 865 picocuries, or about one trillion parts of curie). The human body weighing 70 kg contains about 175 g of potassium, therefore, about 5,400 decays (≈ 5,400 Becquerel) occur every second, moreover, continuously throughout human life.
Becquerel (Russian designation: Bq; international: Bq) is a measure of the activity of a radioactive source in the International System of Units (SI). One becquerel is defined as the activity of a source in which, on average, 1 radioactive decay occurs in 1 second. The unit is named after the French scientist Antoine Henri Becquerel, one of the discoverers of radioactivity.
In principle, there is nothing surprising. In nature, there are more radioactive food, moreover, radioactive, not only because of 40 K, but also, for example, radium (isotopes 226 Ra, 228 Ra). As an example, a Brazil nut is perfectly suited , the radioactivity of which can reach 12,000 picocuries per kilogram and above (450 Bq / kg and above).
To the note : smokers are the worst in this respect, since tobacco contains not only the already mentioned radium 226 Ra, but thorium 234 Th, polonium 210 Po and a whole lot more.
But for some reason, Comrade Gary Mansfield from the Livermore National Laboratory. Lawrence, doing the nuclear security newsletter of RadSafe in 1995, wrote about the “banana equivalent dose” and a new era began. The era of the radioactive banana (the banana equivalent is a much more vigorous thing than the banana argument described in the article ).
The equivalent dose of a banana (BED) is a completely unofficial unit that characterizes the effects of ionizing radiation. Its main purpose is to act as an even standard user available to the standard with which doses of radioactivity can be easily compared. In fact, it is a tool for describing infinitely small doses of radiation (and infinitesimal risks for the population from them). Excerpt from Wikipedia (RU) :
... Since death or a serious illness caused by a low dose of radiation (below 0.5 Gy) is extremely rare, it turned out that it is impossible to link them with radiation effects on the body with confidence - observations will be required for a long time (more than 12 years) over a huge sample people exposed to such a dose. Moreover, the positive effects of low doses of radiation on living organisms, hormesis, were found. The phenomenon of mass consciousness is also associated with small doses of radiation, when uncertainty about the safety issue (or confidence that the existing danger is insignificant) is interpreted as a deliberate danger and a massive fear of small doses of radiation is formed.
A few words about radiation hormesis :
The term radiation hormesis was proposed in 1980 by T. D. Lucky and means the beneficial effects of low doses of radiation. The mechanism of radiation hormesis at the cell level of warm-blooded animals consists in initiating protein synthesis, gene activation, DNA repair in response to stress - the effect of a small dose of radiation (close to the value of the natural radioactive background of the Earth). This reaction ultimately causes the activation of membrane receptors, the proliferation of splenocytes and stimulation of the immune system. (1994 - Report of the UN International Committee on the Effects of Atomic Radiation).
Being a graduate of the Department of High Energy Chemistry, I respect the concept of hormesis (~ radiation hormesis) with respect (respect, in due time, was supported by experimental course work carried out in one of the hospitals). IMHO small but constantly, more harmful than large, but once. Drop stone wears away.
In order to better understand what a small dose and what is NOT a small one, you can use, in addition to the banana equivalent, a visual aid - a summary table of radiation doses ( increase ) created by the engineer and popularizer of science Randall Patrick Monroe (note my banana equivalent is outlined in red) .
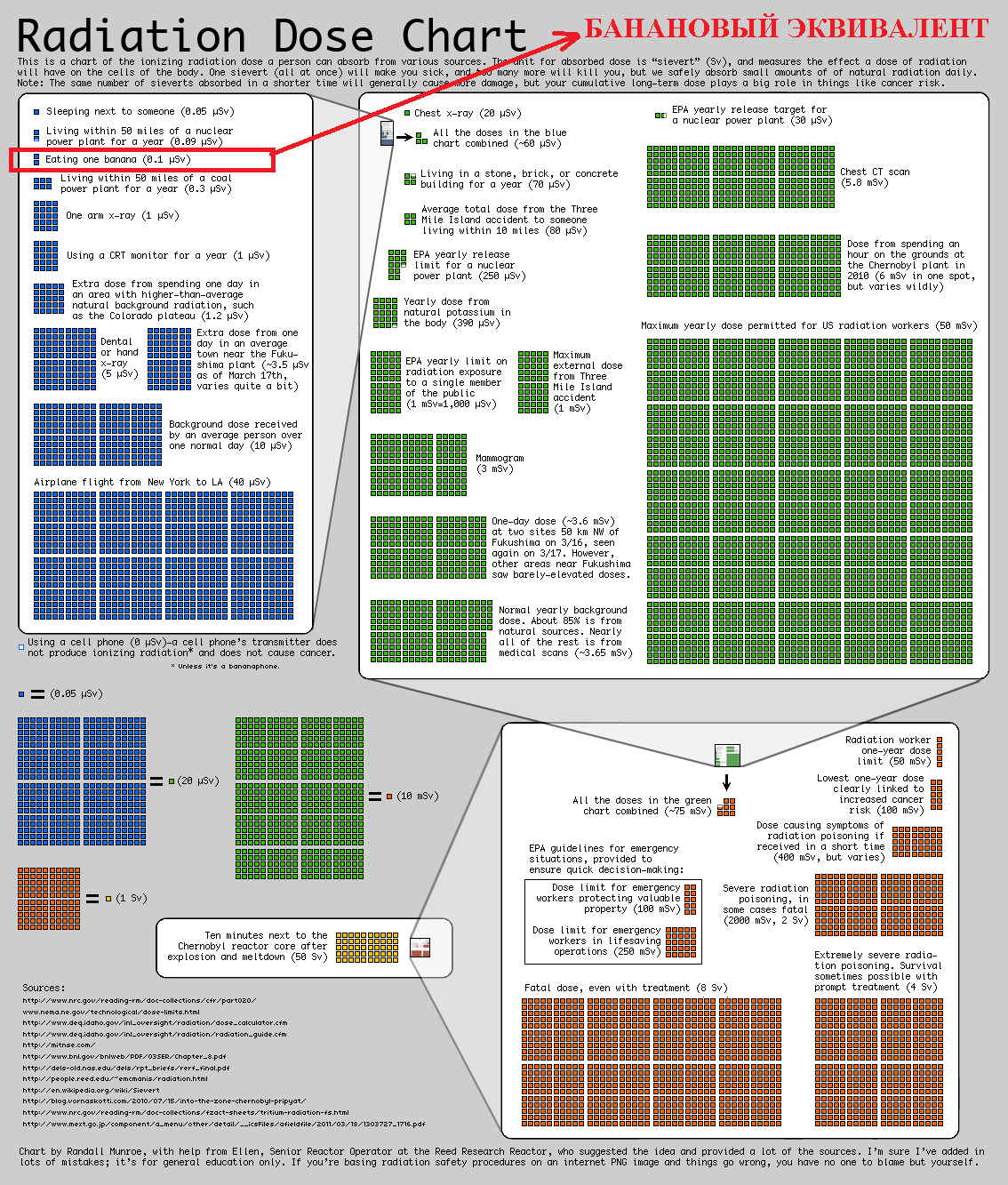
Well, if the table does not suit for some parameters, we return to our banana equivalent. 1 BED is approximately equal to the dose of radioactivity that a person receives by eating one medium-sized banana, weighing about 150 g (5.3 ounces) with an activity of isotopes of about 15 Bq . This is calculated all the way by multiplying the expected equivalent dose that an adult can grab for 50 years from a pure 40 K isotope by the activity of the isotope and by the mass of potassium in a banana. We get:
1 BED ≈ 5.02 nSv / Bq x 32 Bq / g x 0.537 g ≈ 86 nSv = 0.086 µSv (µSv) = 8.6 micro X-rays (μrem)
Basically, it is customary to round this value to 0.1 µSv (10 micro-roentgens) to simplify calculations and ease of perception. In general, if you eat one average banana daily for a year, the total equivalent dose will be ≈ 37 μSv ≈ 3.7 mrem.
By the way, the expected equivalent dose (5.02 nSv / Bq) is taken from American sources ( EPA ). But the International Commission on Radiological Protection uses a different value for this coefficient = 6.2 nSv / Bq and then when recalculating the dial it will turn out not so beautiful. It will be more difficult to count, to represent scales, etc. Therefore, they use American data.
Note: i.e. theoretically, using the above formula can create your% VEGETABLE / FRUIT% equivalent relative to 40 K. For example, the average weight of a commodity tuber of a variety (a bag which Lukashenko gave Putin for the New Year) is 100 grams. We go to watch the base of the US Department of Agriculture on the fact of potassium content in potatoes. It is also important to choose the right option (with skin / without, etc.). Well, let it be on average 430 mg of potassium. We consider and get 6.9 microroentgen. Draw your own conclusions (or do not do it, but read on).
Why is the unit unofficial (and even comic)? And because the "external" potassium (and therefore its isotopes), which is ingested with food, does not accumulate in it (ie, the "banana dose" is not cumulative). This is due to the homeostasis of the human body.
Homeostasis (ancient Greek ὁμοιοστάσις from ὅμοιος “identical, similar” + στ неподвижσις “standing; immobility”) - self-regulation, the ability of an open system to maintain the constancy of its internal state through coordinated reactions aimed at maintaining dynamic equilibrium.
Those. any excess of the component that comes with food is rather quickly compensated by the withdrawal of the same amount with secretions of the body. In fact, the additional radiation caused by consuming a banana lasts only a few hours after ingestion, that is, until the kidneys restore the normal potassium content in the body. This is what the document issued by the US Environmental Protection Agency says . I will quote:
For radioisotopes of elements actively involved in the homeostasis of the human body, the correction factors for calculating the risk of inhalation or ingestion given in this document are not suitable for use in some cases. For example, the risk coefficient of ingestion for 40 K is not suitable for calculation when using natural products containing a high content of 40 K. This is due to the fact that the biokinetic model used in this document implies a relatively slow removal of this element (biological half-life of 30 days) that occurs when the average amount of potassium in the body. A sharp increase in the use of potassium with food leads to the elimination from the body of an equal mass of biogenic potassium (including the isotope40 K) in a short period.
Plus, if the estimated residence time of a certain mass saturated with an isotope in the body decreases N times (due to the simultaneous intake of a laxative, for example), then the calculated equivalent absorbed dose decreases N times too.
So ... A much more harmful phenomenon, in my opinion, is the case when many small sources of radiation are combined (in storage or in warehouses). No wonder there are stories about the false alarms of ionizing radiation sensors at US customs when cars loaded with bananas passed through a checkpoint.
I don’t know how many people know, but Canada, Belarus and Russia are the largest producers of potash fertilizers in the world (!). Most often, these fertilizers are in the form of potassium chloride KCl, Potassium-magnesium K 2 SO 4 * MgSO 4 and rare potassium nitrate KNO 3 . And here already scales far from banana. For example, in 1 kg of the most common potash fertilizer KCl (potassium chloride) ~ 524 grams of potassium, i.e. that's almost 1000 BED (a thousand bananas). Naturally, no one in their right mind will eat this fertilizer, and it will not, because about 15 grams by mouth can easily lead to cessation of heart contractions. But on the other hand, he often saw, especially during the spring sowing campaign in Belarus, the men who adjoined to rest on sacks of fertilizer.

Roughly speaking, he stuffs electrons (we do not take into account the decay with the release of a gamma quantum) back rather quickly (about the effects on the body, thanks to the Javian specification , can be read here ). Polyethylene bag will not save . Below is a picture for those who forgot the lessons of GO (or who simply did not have them :()
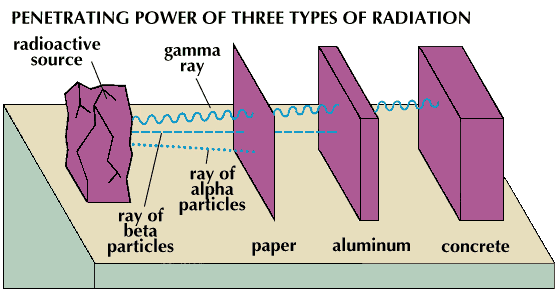
Beta particles (electrons) can more or less be absorbed by only a few millimeters of aluminum. Wrap yourself with foil before you lie down or something ...
In conclusion, as always, a small laboratory work on the subject “we study the dosimeter”. The pictures are a comparison of bananas and some salts containing potassium.

Вот такая банка с китайским КОН (гидроксид калия). Думаю средство для прочистки труб "Крот" шпигует электронами похоже (если там используется KOH, а не более дешевый NaOH)

Дает вот такой фон

But such values in Chinese KCl (potassium chloride)

Well, talking about salt would be incomplete, if you do not mention KBr (the same sedative who is allegedly fed to soldiers in the barracks to reduce libido), the Soviet production is still

The difference, as they say, is visible to the naked eye. So that...
The moral of the note is that the banana radioactivity = the 40 K radioactive isotope existing for thousands of years . If you come from the constellation of Sirius (and all the dogons can confirm this ) with a different level of background radiation, you will have to give up bananas (and from the Belarusian potato, by the way). the rest - "do not think about it." Smoking, by the way, harms much more (for objective reasons, such as the fact that the gamma radiation that occurs during the decay of the isotopes present in tobacco penetrates more than some kind of electron from a banana). Well ... beware of a long stay near large clusters of bananas / potassium salts, etc. sources 40 K.
Sergey Besarab ( Siarhei V. Besarab )
Under the spoiler comments from Sumah about the banana equivalent
Лукавство бананового эквивалента
- Калий-40 — это высокие энергии со всеми вытекающими. энергии излучения могут быть разными у разных изотопов. если вместо калия будет изотоп с низкими/средними энергиями, вред не обязательно будет таким же или меньше. На коротких расстояниях внутри организма ещё вопрос, что хуже/лучше высокие энергии или низкие.
- Рассуждения о банановом эквиваленте касаются в основном внешнего облучения. Тайваньский пример — это внешнее облучение. Жители здания не вдыхали кобальт, не ели кобальт. Но даже при внешнем облучении нужно учитывать, что энергии бывают разные.
- Рассуждения о банановом эквиваленте как об эквиваленте внутреннего облучения — это тоже очень странно. У разных изотопов разные периоды полувыведения из организма. В нашей среде есть изотопы, выведение из организма которых не доказано. А значит при любой концентрации этих изотопов в среде они накапливаются. Т.е. порога для не существует. Сколько организм натянет из среды этого %@#ма, столько и останется в могиле и/или частично вылетит в трубу крематория.
- Никто не отменял разную радиотоксичность изотопов. СанПины никто не отменял.
- Никто не отменял локальную концентрацию радиоактивных изотопов в организме по сравнению со средой обитания, которая может отличаться и в 10, и в 100 раз. Никто не отменял локальную концентрацию по организму, которая так же может отличаться на порядки.
- Энергии калия-40 и энергии космического излучения (в самолёте) — это тоже как бы разные энергии.
- Про примеры с дозиметром. Газоразрядные дозиметры завышают мощность дозы, находясь в поле высоких энергий. Мощность дозы не может быть мерилом безопасности.
Про видимость калия дозиметром в продуктах питания и прочих «чудесах», связанных с обнаружением радиоактивных изотопов в продуктах питания и в среде.
Если использовать сцинтилляционый детектор цезий йодный размером 5530 и свинцовую камеру с толщиной стенок 6мм (дно камеры 12мм), то результаты будут следующими при статистической погрешности 2% и одной сигме (доверителный интервал 68%):
— при естественном фоне около 10мкР/ч внутри пустой свинцовой камеры сцинтиллятор насчитает 0,070мкР/ч.
— если положить в камеру небольшой образец продукта, например, не самый жирный питьевой йогурт в зип-пакетик налить, то на поверхности пакетика дозиметр насчитает около 0,080мкР/ч или побольше.
Разница в показаниях будет обусловлена влиянием калия.
Ирония заключается в том, что в йогурте в этот момент вполне может быть цезий-137 на уровне единиц Беккерелей на литр, но он никак не проявится. Т.е. излучение от радиоактивного калия будет полностью маскировать излучение от радиоактивного цезия-137.
В магазинах Дикси продаётся йогурт клубничный в тетрапаке 0,5 литра с содержанием цезия-137 на уровне примерно 4Бк/литр. Йогурт производится в населённом пункте Стародуб Брянской области. Населенный пункт Стародуб находится на границе довольно существенного чернобыльского пятна в этом регионе. Ну, и Брянская зона отчуждения не очень далеко от Стародуба.
4Бк/л — это, конечно, ниже нормирования по цезию-137, но если вы сидите на субстрате из цезия-137 непрерывно, то рассуждения о полувыведении цезия-137 из организма становятся неуместными для объяснения безопасности такого потребления.
Кроме того, можно считать, что цезий-137 — это маркер радиоактивного загрязнения, о котором мы мало что знаем.
Полно примеров, когда на воздухе гамма-спектрометр не обнаруживает пик цезия-137, а обнаруживает какофонию в области низких энергий. При этом в образце поверхностного грунта или гриба/ягоды гамма-спектрометр из техногенных изотопов обнаруживает только следы цезия-137. Причём этого цезия может быть 50Бк/кг и больше
Как повысить уровень детектирования "в быту", если такое вообще возможно?
— можно каким-то образом увеличить удельный вес радиоактивного вещества: сублимировать образец продукта, к примеру.
— если есть сцинтиллятор, можно использовать свинцовую камеру или экран в виде толстого слоя воды или снега, экранирующих излучение от земли.
— если торцевой дозиметр, тогда свинцовую камеру.
— если газоразрядный дозиметр, то сублимация образца. экраны тоже можно использовать, но газоразрядник очень чувствителен к космическому излучению. оно будет периодически портить картинку чаще, чем необходимое время экспозиции. в принципе, если записывать график мощности дозы, можно попробовать просто вырезать точки, похожие на реакцию на космическое излучение, но это всё танцы с бубнами. но экран позволяет газоразрядником оценить обстановку быстро. падение мощности дозы над экраном всё равно будет. бетон, кстати, неплохо экранирует, если гравий внутри бетона не светится сильно.
Но из всего вышеперечисленного сцинтиллятор и свинуовая камера — самое эффективное и быстрое. чтобы добраться до результатов 0,070мкР/ч с погрешностью 2% нужно часа два экспозиции

