The tale of the alloy Rose and fallen off

A long time ago, when I was a schoolboy and minted radio components mainly from various boards thrown into a dump, I noticed an unusual phenomenon in the process of unwinding another such board: some rations instantly fell off the foil, it was necessary to poke a soldering iron at them. The contact pad remained clean from the solder, smooth and silver-trimmed, and a drop of solder on the output part had the same shiny flat base at the bottom.
Noticed and forgotten for the time being. And the year before last, taking part in a scientific expedition to the Arctic, I unexpectedly encountered an unexpected failure of the device with which I worked. The device was self-made - it was made by other people, but fortunately they provided me with a scheme and all the documentation, I took with me just in case a soldering iron and the necessary devices. There was no need to search for a long time failure: inside the case there was an integrated 5-volt stabilizer in the D-Pak case, which simply fell off the board. The contact pads and the “belly” of the stabilizer had the same beautiful shiny surfaces.
The last case was with an old laptop, which, according to its former owner, had a power connector changed in a basement for a thousand rubles after the old one had stopped contacting. Over time, the contact in this connector again had problems, and I found that the connector was just poorly soldered and it just hung in the board, picked up and disappeared the connector, as it should. But time passed and the malfunction returned.
As you guessed, the reason for all these phenomena is the same, and it is mentioned in the title of the article and is shown on CDRV. But where did it come from on boards and even in a laptop?
In the first two cases, someone's rationalization, which at some point became the almost accepted way of tinning printed circuit boards at radio amateurs, and apparently penetrated into production, was to blame for everything. He threw a board into a mixture of water, glycerin and citric acid, heated to one hundred degrees, threw some Rosa alloy pellets into it, dispersed the melted alloy with a rubber spatula - that's what beautifully served and easily soldered tracks are ready for. And, as we remember, the laptop was visited by unofficial repairmen, who have one nice trick - how to solder soldered to massive board polygons, and also lead-free solder, a frail soldering iron. For this purpose, the same alloy is used for the Rose, which, when fused with a tight lead-free metal, quickly melts it and makes it easy to dismantle the connector, without “blackening” everything around the board and without peeling the copper from the PCB.
It would seem that a little Rosa alloy should not change the properties of the solder very much. But it is not. Why - let's remember that the Rose alloy is a triple eutectic in the tin-lead-bismuth system.
Let's talk about eutectic

Let's look at the phase diagram of a two-component system with unlimited solubility in a liquid state and insignificant solubility in a solid one. The composition of the alloy is plotted on the horizontal axis, and the temperature on the vertical axis. And the lines on it are dependences of the temperatures of the beginning of melting (solidus - ADCB) and the end of melting (liquidus - AEB). There are still two branches separating the regions of a homogeneous solid solution from the two-phase region, but they will not interest us now. In the area between the solidus and the liquidus, we have a two-phase system from the melt and the solid phase.
Point E is a special one, in it the solidus and liquidus touch each other: the alloy of such composition is the most fusible and it melts at once, like a pure metal. This is eutectic. A good solder is usually just a eutectic, and this is precisely POS-61 or POS-63.
And if the composition of the alloy does not match the eutectic? Have you ever soldered with POS-40 solder, which was usually sold in Soviet hozmagi in the form of a thick bar? Under the tip of the soldering iron, he first turns into a kind of porridge, and then only melts completely. It hardens in the reverse order, first turning into a mess, and then frozen completely.
And if we take tin and add only 5% lead to it? It will be absolutely the same, only between the solidus and the liquidus “porridge” will be almost solid. But fragile, since the liquid phase will fill the thin layer between the crystals.
And now notice that the solidus line is horizontal. This means that the melting of any alloy of tin and lead (in the range of compositions of 2.6-80.5% lead) will begin at the same temperature , regardless of its composition. At the same temperature, solidification will end, and by the way - the composition of these last melt drops is equal to the composition of the eutectic.
Now let's add bismuth legs
And if you add a third component, which also freely dissolves in a liquid state, but does not dissolve in a solid ... Here we need to consider a three-component system.
In general, such a system behaves similarly to a two-component one. Here, too, there is a composition of three components, where the solidus and liquidus temperatures are equal. And its melting point is even lower than the temperatures of binary eutectics in each of the three binary systems that make up the triple one.
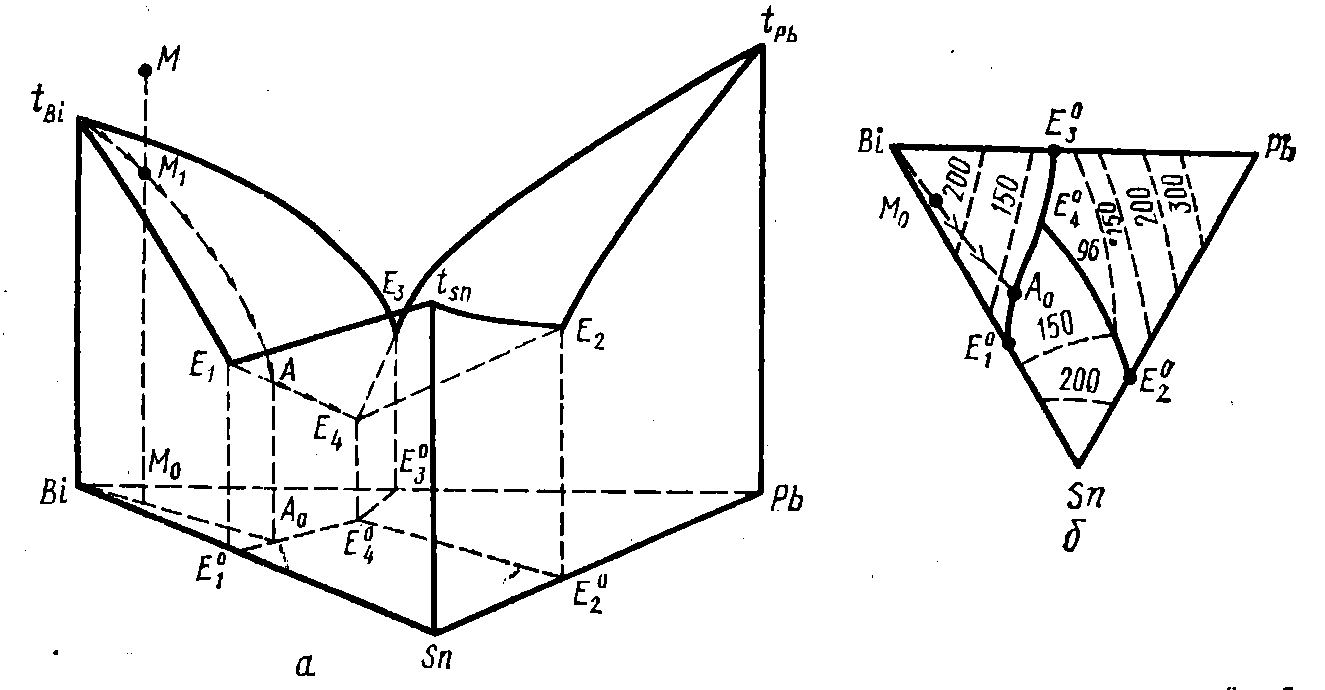
This figure shows the liquidus, which turned from a line into a surface. And the solidus ... Solidus is a horizontal plane for almost the entire triangle (except for the lead angle - there is an intermetallic phase). For the lead-tin-bismuth system, its position corresponds to a constant temperature of 96 ° C — the melting point of the Rose alloy.
So if we add a little bismuth to the tin-lead alloy, we get an alloy that begins to melt at 96 ° C.
True, bismuth is noticeably soluble in tin, and especially in lead. Because of this, the solidus plane is moved away from the edge of the triangle - a tin-lead cut. It is about 15% bismuth away from the eutectic tin-lead, “bending” up when approaching the edge. Therefore, the amount of alloy Rose, which will lead to trouble - not infinitely small, but about 10-20%. But unfortunately, this is only in ideal conditions. In real and hurt a smaller amount. The reason for this is that soldering is a quick process.
Kinetic factor
Kinetics is a section of chemistry dedicated to the speed of chemical processes. Soldering is a quick and short-term process, the soldering point heats up quickly before the solder melts and cools quickly. What does this lead to?
Imagine a contact pad on the board, decorated with Rosa alloy (specifically or after using this alloy to ottap the defective part). The solder pad was soldered to it and the soldering iron removed. Solder froze. Soldering time - seconds. During this time, the solder and Rosa alloy will not have time to mix, especially if the SMD-element is soldered and the narrow gap between the contact pad and the output pad prevents mixing. As a result, on the site of the former Rosa alloy, a layer of a bismuth-rich layer is obtained on the contact pad, which begins to melt at a temperature of 96 ° C, even if the total amount of contaminating bismuth junction would seem to be insufficient. It was for this reason that the parts fell off the light touch of the soldering iron, and therefore a “mirror” was formed.
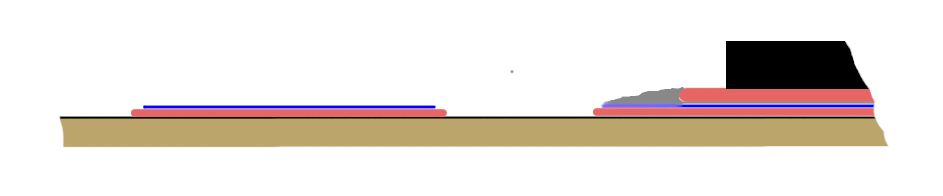
The blue in this figure shows the Rosa alloy, and the gray one shows the solder. Left - before, and right - after soldering.
What threatens?
When the heated component is soldered with solder to the Rose alloy, the result is clear: the component will simply fall off. At temperatures above 96 ° C, the crystalline solder grains are separated by liquid interlayers and its strength is similar to that of wet sand. It would seem that if the part is not heated, there is nothing to fear? But here comes the factor that from the time of soldering to the moment of final solidification a lot of time passes. And at this time, the slightest effort to collapse it will destroy, there will be cracks. It turns out a kind of "false soldering": everything seems to be soldered, there is a contact - but there is no reliability, over time this contact will disappear, especially under mechanical loads, like on a laptop's power connector.
findings
Do not use the Rosa alloy for tinning the boards or for watering the parts. And if you need to solder Rosa some delicate and very afraid of overheating part, get yourself a separate soldering iron or a separate tip for this. A worthy alternative to the Rosa alloy tinning is chemical tinning. Just be sure to put on the "chemical" tin flux and melt it.
When the part is not mechanically loaded and you nevertheless soldered it with the Rose alloy (or someone did it before you), do not be lazy and glue it to the board before soldering. This way you to some extent insure it against displacement during the solidification of the solder and make the soldering more reliable. You can also walk around the sites with the Rosa alloy with a large drop of solder on the soldering iron's wide tip, then remove the solder with braid and repeat this operation 1-2 times more, but depending on the quality of the board there is a risk that the tracks will not withstand.
PS:
A similar situation arises if you suddenly encounter tin-bismuth solder. Such a solder, being of low toxicity (bismuth is much less toxic than lead) and low-melting (Tpl = 139 °), would be an excellent lead-free solder if it were not for the formation of a triple eutectic upon ingestion of lead. For example, when repairing a board that is soldered in this way using ordinary tin-lead solder. However, such a solder, as indicated by Habra_nik , has a certain level of popularity in Japan. So you need to be careful when repairing modern Japanese electronics.
