Generic Medicines and Competition
An article from the British journal The Economist on August 6, 2009.
Especially for the Pirate Party of Russia
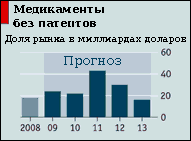 Since at the moment, the governments of many countries are having difficulty paying medical bills, they are starting to pay more attention to the prices of medicines. This may seem unfair, given that pharmaceuticals account for only a fifth of the total cost of medical care, as well as the fact that innovation can fade if it is not funded. Nevertheless, there is an opinion that large manufacturers overestimate the prices of drugs too much and suppress independent producers who produce similar drugs cheaper. The governments of Canada and Japan have shown interest in this matter. And this time they really have something to criticize.
Since at the moment, the governments of many countries are having difficulty paying medical bills, they are starting to pay more attention to the prices of medicines. This may seem unfair, given that pharmaceuticals account for only a fifth of the total cost of medical care, as well as the fact that innovation can fade if it is not funded. Nevertheless, there is an opinion that large manufacturers overestimate the prices of drugs too much and suppress independent producers who produce similar drugs cheaper. The governments of Canada and Japan have shown interest in this matter. And this time they really have something to criticize.
The most egregious offense is transactions, the essence of which is that the patent holder pays independent producers to delay the production of a cheaper copy. The US Department of Justice called such deals "allegedly illegal" and promised to actively pursue them. The Federal Trade Commission assumes that they (transactions of a comment of the translator) will cost American citizens $ 3.5 billion a year.
The US government is looking for other ways to regulate. One option is a law that should encourage the production of generic drugs, the essence of which is that it guarantees six months of monopoly to the manufacturer, who will be the first to obtain registration of a drug on the basis of a patent in the USA. However, often they do not have complete freedom because patent holders began to produce “official” generic versions under well-known brands. Large manufacturers claim authorized independent companies are cutting prices. Good, but they also undermine the meaning of the six-month bonus and, therefore, the incentive for independent companies to win it.
presidents in the European Union are also concerned about this issue. The EU Commission has calculated that independent companies begin production seven months after the patent expires; such delays cost consumers more than 3 billion euros per year. The Antimonopoly Commission opposed such transactions, they also did not support all other methods that large companies use, including filing hundreds of patents for one medicine, to prevent potential independent competitors. Neil Kros, EU Commissioner for Antitrust, said “something is wrong” in the industry.
Large manufacturers have two excuses: in reality, everything is much more complicated in comparison with how antitrust organizations describe it, and also that the “pay for delay” system is actually more beneficial for the consumer. Yes, indeed, the real picture is ambiguous, this is not the fault of all the major drug manufacturers, but the second excuse is fundamentally wrong.
In the EU, generic medicines were not welcome, unlike America, Europe consists of small markets with different regulations and economic barriers, one of them is “the requirement to register the medicine in all EU countries”. Available evidence suggests that a unified European patenting system, as well as relevant judicial procedures, together with the support of leading companies, would increase the number of generic medicines.
Firms selling non-proprietary funds often seek market access by challenging existing patents rather than trying to wait for their expiration dates. A single patent, and therefore a single problem, could simplify their lives.
American independent companies are not angels either, they are happy to receive money for deferring the start date of production. They also say that this is best for the consumer in that it reduces the number of legal inconsistencies, as well as contributes to a more rapid appearance of the drug on the market.
As recent manufacturers deals show, this is all nonsense. Some third-party manufacturers also agree to delay the start of sales, not for the sake of money, but for the promise of the patent holder to delay or cancel the start of their own sales. Such frauds may possibly suit brand owners, as well as manufacturers of generic drugs that can wreak havoc in other ways. But neither one nor the other is worried about the financial condition and health of the consumer.
_________________
Translator’s note
In the original text the word “generic” is often used, which translates as “patent-free”, I decided that when it is used in combination with the word “company” it is better to translate it together as “independent company”, though I I could be wrong.
PS This is my first habrotopik =). Do not judge strictly plz ^ _ ^.
Special thanks to my proofreaders =)
OlegXxl and Stixoplet92
Especially for the Pirate Party of Russia
Authorities should stop using a system that delays the appearance of generic medicines
 Since at the moment, the governments of many countries are having difficulty paying medical bills, they are starting to pay more attention to the prices of medicines. This may seem unfair, given that pharmaceuticals account for only a fifth of the total cost of medical care, as well as the fact that innovation can fade if it is not funded. Nevertheless, there is an opinion that large manufacturers overestimate the prices of drugs too much and suppress independent producers who produce similar drugs cheaper. The governments of Canada and Japan have shown interest in this matter. And this time they really have something to criticize.
Since at the moment, the governments of many countries are having difficulty paying medical bills, they are starting to pay more attention to the prices of medicines. This may seem unfair, given that pharmaceuticals account for only a fifth of the total cost of medical care, as well as the fact that innovation can fade if it is not funded. Nevertheless, there is an opinion that large manufacturers overestimate the prices of drugs too much and suppress independent producers who produce similar drugs cheaper. The governments of Canada and Japan have shown interest in this matter. And this time they really have something to criticize.The most egregious offense is transactions, the essence of which is that the patent holder pays independent producers to delay the production of a cheaper copy. The US Department of Justice called such deals "allegedly illegal" and promised to actively pursue them. The Federal Trade Commission assumes that they (transactions of a comment of the translator) will cost American citizens $ 3.5 billion a year.
The US government is looking for other ways to regulate. One option is a law that should encourage the production of generic drugs, the essence of which is that it guarantees six months of monopoly to the manufacturer, who will be the first to obtain registration of a drug on the basis of a patent in the USA. However, often they do not have complete freedom because patent holders began to produce “official” generic versions under well-known brands. Large manufacturers claim authorized independent companies are cutting prices. Good, but they also undermine the meaning of the six-month bonus and, therefore, the incentive for independent companies to win it.
presidents in the European Union are also concerned about this issue. The EU Commission has calculated that independent companies begin production seven months after the patent expires; such delays cost consumers more than 3 billion euros per year. The Antimonopoly Commission opposed such transactions, they also did not support all other methods that large companies use, including filing hundreds of patents for one medicine, to prevent potential independent competitors. Neil Kros, EU Commissioner for Antitrust, said “something is wrong” in the industry.
Food for thought
Large manufacturers have two excuses: in reality, everything is much more complicated in comparison with how antitrust organizations describe it, and also that the “pay for delay” system is actually more beneficial for the consumer. Yes, indeed, the real picture is ambiguous, this is not the fault of all the major drug manufacturers, but the second excuse is fundamentally wrong.
In the EU, generic medicines were not welcome, unlike America, Europe consists of small markets with different regulations and economic barriers, one of them is “the requirement to register the medicine in all EU countries”. Available evidence suggests that a unified European patenting system, as well as relevant judicial procedures, together with the support of leading companies, would increase the number of generic medicines.
Firms selling non-proprietary funds often seek market access by challenging existing patents rather than trying to wait for their expiration dates. A single patent, and therefore a single problem, could simplify their lives.
American independent companies are not angels either, they are happy to receive money for deferring the start date of production. They also say that this is best for the consumer in that it reduces the number of legal inconsistencies, as well as contributes to a more rapid appearance of the drug on the market.
As recent manufacturers deals show, this is all nonsense. Some third-party manufacturers also agree to delay the start of sales, not for the sake of money, but for the promise of the patent holder to delay or cancel the start of their own sales. Such frauds may possibly suit brand owners, as well as manufacturers of generic drugs that can wreak havoc in other ways. But neither one nor the other is worried about the financial condition and health of the consumer.
_________________
Translator’s note
In the original text the word “generic” is often used, which translates as “patent-free”, I decided that when it is used in combination with the word “company” it is better to translate it together as “independent company”, though I I could be wrong.
PS This is my first habrotopik =). Do not judge strictly plz ^ _ ^.
Special thanks to my proofreaders =)
OlegXxl and Stixoplet92
