Goodbye cold: the introduction of drugs into the pockets of viruses

Outside the window, the bright sun shines, the air is filled with warm moisture, and the cool breeze decided to take a vacation, leaving us to the torn heat. I want to change my jeans to shorts, a laptop to a volleyball, and an office to the beach. Summer is associated with holidays and vacations, but sometimes a guest whom we usually expect in the winter months, namely a cold, is squeezed into this idle list. There is nothing worse to temperature when the air temperature is about 30. In the summer, acute respiratory viral infections turn a person into a snail, slowly weaving along hot asphalt, leaving behind a mucilaginous trail.
Can rescue science help us fight rhinoviruses, you ask? Of course, maybe scientists will answer you if they discover a new way to deal with rhino / enteroviruses hidden in the previously unexplored “pocket” of the virus. What kind of pocket is this, what do scientists want to put into it, and how will this help to defeat rhinoviruses? We will find the answers in the report of the research group.
Study basis
To begin with, we quickly recall from what we have ARVI and what it really is.
Visualization of rhinovirus.
ARVI, that is, an acute respiratory viral infection, is the result of the active activity of the virus in the human body, namely in its respiratory system. These viruses include rhinoviruses from the genus enteroviruses. One of the main structural features of rhinovirus is the open virion, that is, a virus particle without a shell.
In humans, rhinoviruses cause inflammation of the upper respiratory tract, because they prefer to multiply in the mucous membrane that covers the nasopharynx. Symptoms in case of infection are fairly standard and familiar to everyone - temperature, runny nose, pain in the larynx. In the absence of treatment and a decreased immune response of the body, acute respiratory viral infections can develop into bronchitis, sinusitis or otitis media.
Can rhinoviruses be treated and / or vaccinated? Theoretically possible, but almost impossible, since the number of serological variants of the pathogen is very large. Rhinovirus infections are treated by alleviating the symptoms, and the virus itself defeats the immune system.
If we talk about the treatment of a viral infection as such, then it is worth understanding the process of killing the virus itself. As a rule, this is carried out by introducing a drug into it, roughly speaking. At the moment, the most studied inhibitors are capsid binders, which are placed in the hydrophobic pocket of the viral capsid.
In the study we are considering today, scientists have identified a previously unknown pocket that is formed by the viral proteins VP1 and VP3 and is a common feature for different types of entero-rhinoviruses. The introduction of the drug into this pocket will stabilize the key region of the virion, which will prevent the conformational expansion necessary for the release of viral RNA. Therefore, no snot, cough and fever. Agree, it sounds very inspiring.
Research results
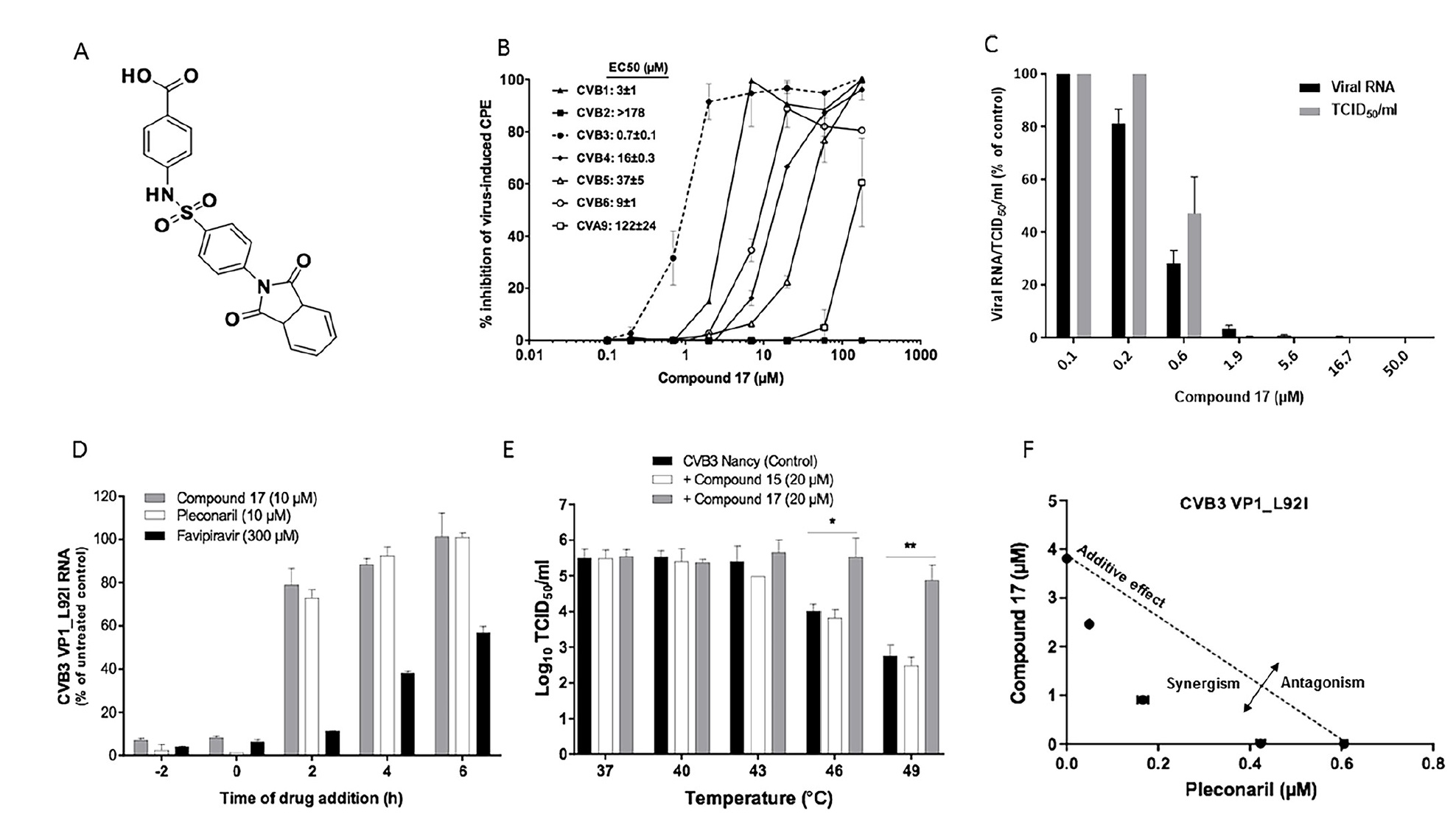
Image No. 1
Scientists have found that compound 17 ( 1a ), which is a derivative of benzenesulfonamide, is a fairly powerful inhibitor for the B3 strain of Coxsackie virus. The half-maximum effective concentration (EC50) is 0.7 ± 0.1 μM ( 1b ).
Compound 17 also inhibits the replication of CVB1 and CVB6, exhibits moderate activity against CVB4, CVB5 and Coxsackie A9 virus (CVA9), but does not react against CVB2 even at the highest concentration tested ( 1b ). Also, this compound is not inhibited in CVA16 and EVA71 (group EV-A), CVA21 and PV1 (group EV-C), EVD68 (group EV-D) and rhinovirus B14 (RVB14, RV-B).
The antiviral activity of compound 17 was additionally confirmed in a viral load analysis, in which it reduced the dose-dependent manner of CVB3 and CVB3 virus RNA formation with EC50 values of 0.4 ± 0.01 μM and 1.1 ± 0.3 μM, respectively ( 1 s ).
Compound 17, like pleconaril (an antiviral drug), targets the replication of CVB3 in the early stages of the viral cycle. In the case of the introduction of compound 17, 2 hours after infection, the antiviral activity is greatly reduced ( 1d ).
Scientists remind us that early stage enterovirus inhibitors (e.g. pleconaril) interact with viral capsids and increase their resistance to thermal inactivation. Compound 17 increased the thermal stability of CVB3 by 1.5 at a temperature of 46˚C and by 2.1 at 49˚C (1e ).
If compound 17 and pleconaril are combined, then a synergistic antiviral activity will be observed. That is, these two substances have different mechanisms of antiviral action ( 1f ).
Observation data allow us to conclude that compound 17 is well suited for tests related to the detected pocket in the viral capsid.
In order to better consider the interaction process of compound 17 and the viral capsid CVB3, scientists conducted cryoelectronic microscopy.
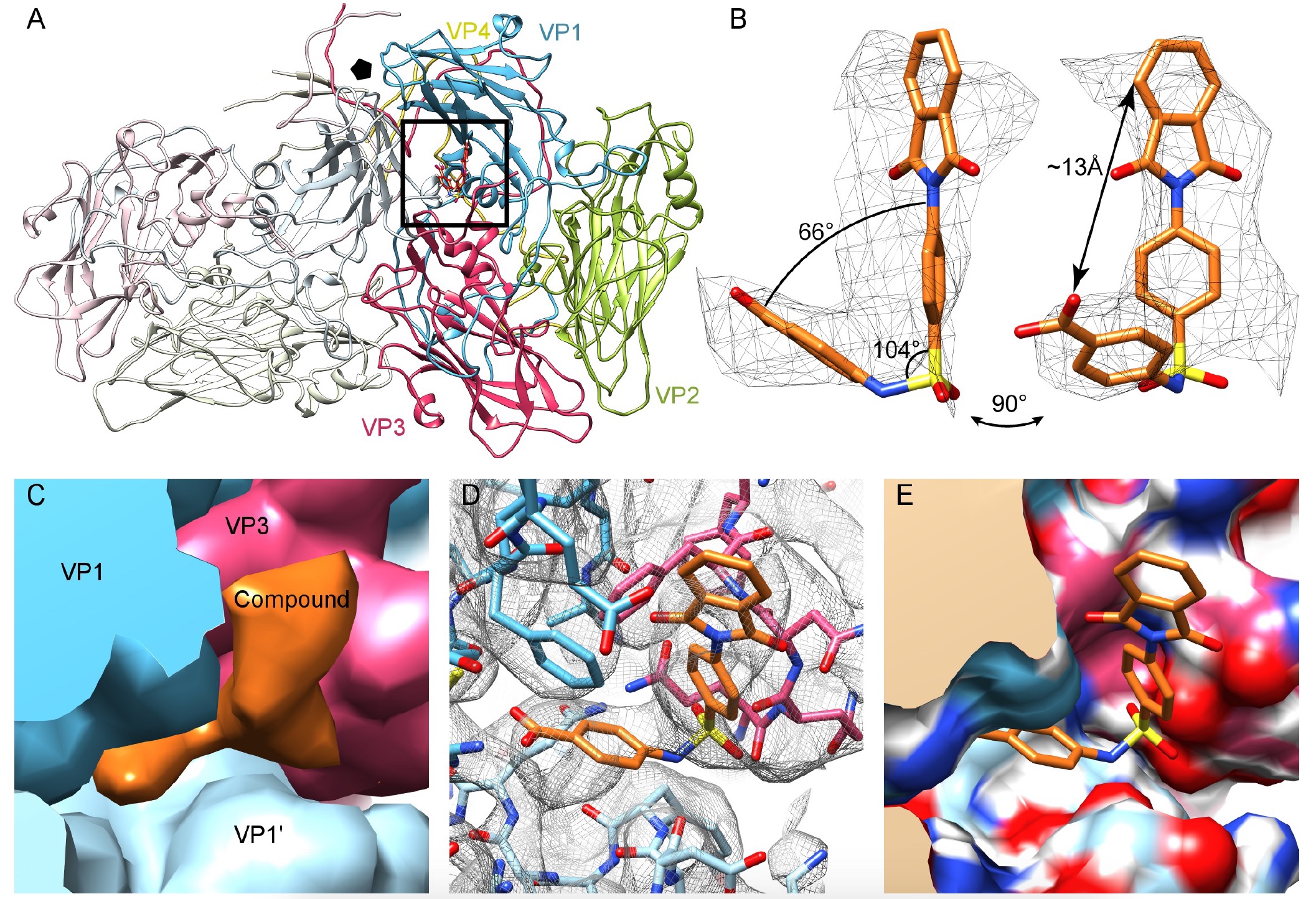
Image No. 2
The capsid protein side chains were easily identified, and the Nancy CVB3 homology model could easily fit into the seal map. In addition, the cryo-EM strain of Nancy showed a lack of lipid factor compaction in the hydrophobic pocket of VP1. However, an analysis of the differences revealed additional compaction, which can be associated with the atomic model of compound 17 ( 2a , 2c ).
As can be seen in image 2b, the compound has an L-shape with a long and short shoulder, which allows for “docking” with connection 17. Simulation showed that connection 17 is connected in the pocket by means of two VP1 blocks and one VP3 block on the protoprotomer interface. Due to the icosahedral type of symmetry of the virus, there can be 60 such regions in one caspid ( 2c ).
The region under consideration is located at a distance of 16 Å from the hydrophobic pocket that the pleconaril is aimed at. Thus, compound 17 does not work in the same place as pleconaril, that is, without interfering with its antiviral activity, but supplementing it.
Scientists have simulated interactions within proteins, interfaces, structures and compounds, which showed the residues VP1 (73, 75–78, 155–157,159–160, 219 and 234) and VP3 (233–236), which form a pocket ( 2d and 2e ) .
An analysis of the conservation of these pocket residues in the 56 amino acid sequences of CVB3 showed that 7 out of 16 are completely preserved (i.e., there is a compound 17 inside), and about 14 residues showed a result of 97% conservation. Pocket preservation also occurs in all enteroviruses of group B.
However, not all viruses are so easily susceptible to inhibition by compound 17. Therefore, it was necessary to understand what makes them so resistant. To do this, scientists carried out clonal selection, highlighting 4 mutations of resistance. Three of the four amino acid mutations were located in close proximity to the pocket (F76C, E78G and A98V), and another (D133G) was quite distant.
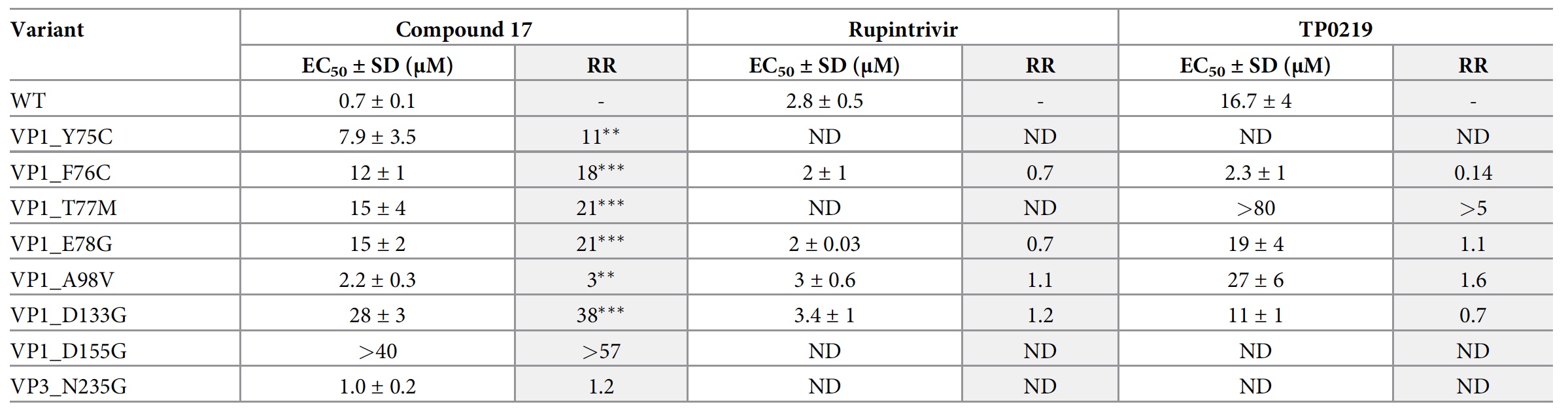
Extent of effect of compound 17, rupintrivir (3C protease inhibitor) and TP0219 (glutathione-depleting compound) on CVB3 WT replication and modified CVB3 variants.
Scientists have prepared 12 CVB3 mutants in the CVB3 clone through reverse engineering. A glutathione-independent variant VPB1_T77M CVB3 has also been created which is resistant to the glutathione-depleting compound TP0219.
Only eight of the 12 mutants were viable (2d ), 7 of them were resistant to compound 17 and react equally to rupintrivir.
Examination of the only resistant mutation far from the pocket has shown that VP1_D133G is more heat resistant than CVB3 Nancy, and compound 17 can still stabilize it. That is, VP1_D133G is a compensatory mutation, and not a mutation that prevents the binding of compound 17.
But VP1_T77 was involved in the binding of glutathione. Analysis showed that the glutathione-independent variant VP1_T77M CVB3 has a reduced sensitivity to compound 17. It was also found that glutathione ethyl ester had no effect on the antiviral activity of compound 17 even at the highest concentration tested.
Next, scientists checked the degree of potential effect of compound 17 on the binding of viral receptors. For this, the protein of the human Coxsackie virus and the adenovirus receptor (hCAR) linked in a chain were used. The binding affinity of CVB3 did not decrease in the presence of compound 17 even at the highest concentration tested. In addition, compound 17 was active against CD55-dependent * enterovirus B (E-11 virus).
CD55 (complement decomposition acceleration factor) * - membrane protein, complement system inhibitor.The experimental data are also confirmed by the fact that the binding sites of the Coxsackie receptor + adenovirus and CD55 + CVB3 do not intersect with the pocket.
Thus, scientists have found that compound 17 integrates perfectly into the capsid pocket of various viruses. The next stage of the study is to check the effectiveness of the drug introduced into this pocket.
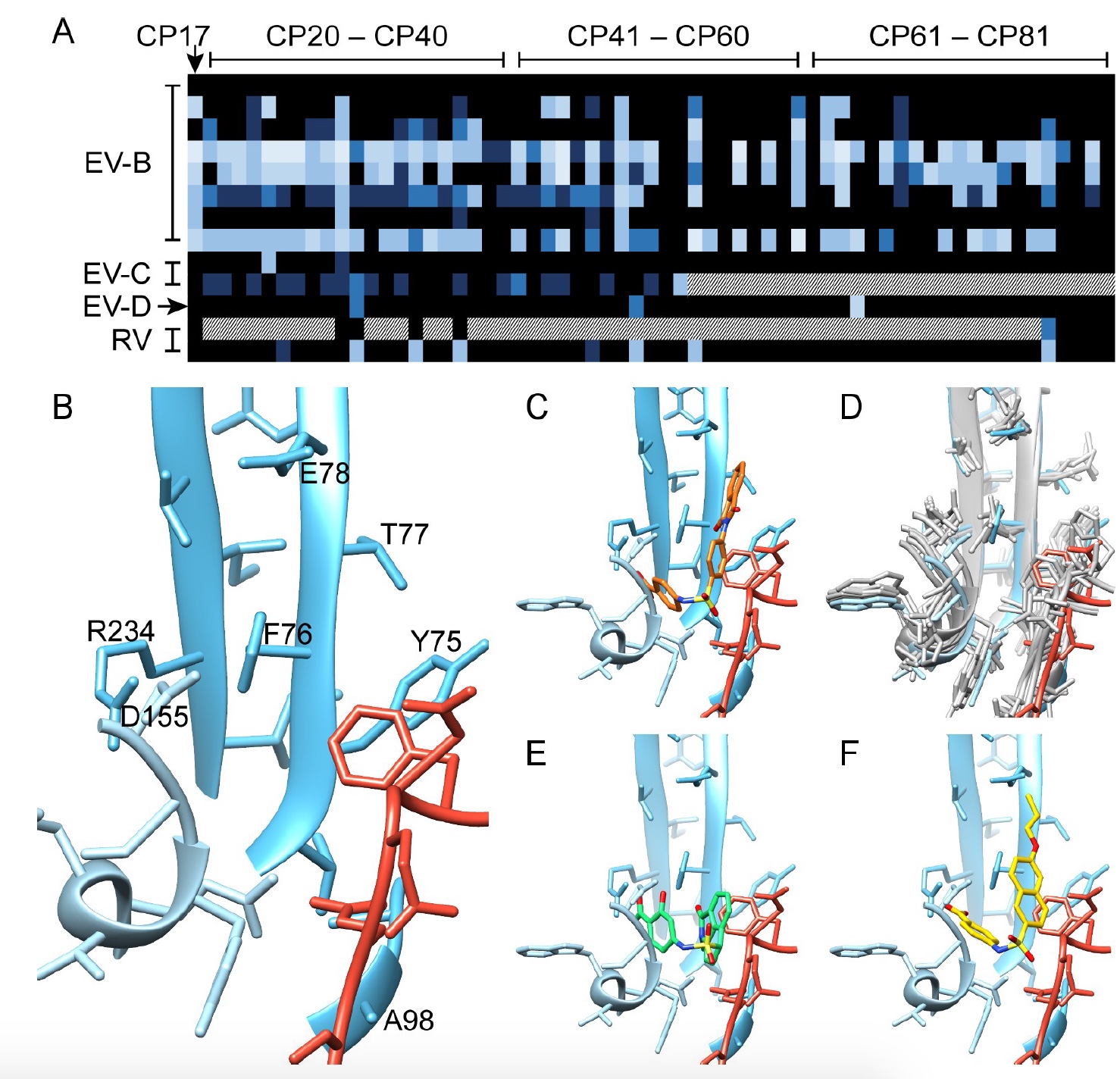
Image No. 3
During this test, we used both commercially available substances (compounds 20-50) and modifications made in the laboratory — compounds 51-81.
Knowledge regarding the activity of compound 17 made it possible to isolate the specific characteristics necessary for the future preparation. Scientists analyzed information from antiviral assays and used it in the synthesis of more active analogues of compound 17.
The synthesis resulted in analogues that were active against a number of enteroviruses: EV-B (CVB), EV-C (PV1 and CVA21), EV-D (EVD68), RV-A (RVA09, RVA59 and RVA63) and RV-B ( RVB14) ( 3a ). Unfortunately, none of the compounds created were active against EVA viruses (CVA16 and EVA71).
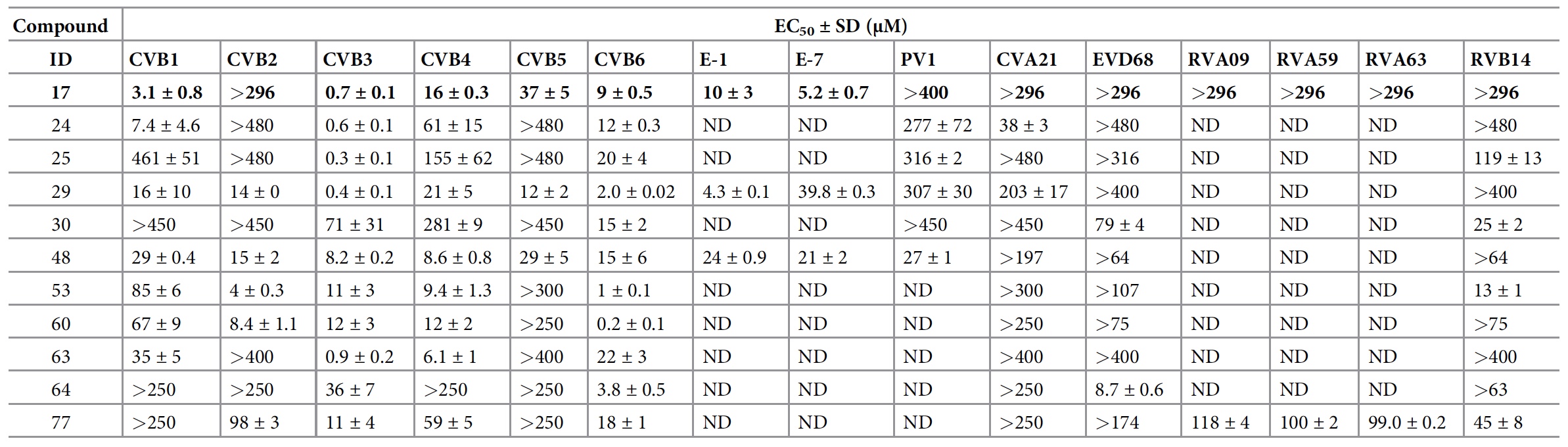
Compounds with maximum potential activity with respect to certain viruses.
Among all analogues, compounds 29 and 48 were active against all 6 tested CVBs (Koskashi group B viruses). Compound 48 completely inhibited PV1 replication at a concentration of 144 μM. Compound 77 showed antiviral activity against RV-A and RV-B.
As seen in image No. 3, the shape of the new molecules complements the shape of the pocket. Scientists were able to establish a clear structure-property relationship (the chemical structure of the molecule to its biological activity) from the carboxyl group in position R3, where the hydrogen atom is necessary for antiviral activity, and in position R2, where the hydroxyl group demonstrates its benefit for a broad antiviral effect. Cryo-EM structuring showed that there is complementarity of charge between R3 and the inhibited residue VP1_R234.
The totality of the results of the tests indicates a fairly high degree of implementation of the tested compounds in the pockets of capsid viruses from various groups.
For a more detailed acquaintance with the nuances of the study, I recommend that you look into the report of scientists ( here orhere )
Epilogue
Summing up, scientists revealed pockets in the structure of enteroviruses that can be filled with drugs that prevent the spread of the virus or completely destroy it.
The main character was compound 17 and its modified analogues, which showed a high degree of activity against CVB3. Identified pockets are quite similar to each other in different groups of viruses, which allows the further development of drugs with a wide spectrum of action.
In other words, viruses that no one has previously treated can now be easily overcome. Of course, relying solely on the human immune system is not the most sensible idea, given the number of diseases and abnormalities that can disrupt its work, opening the doors to the human body to a wide variety of viruses. Such research and development is extremely important in our time, given the population of the planet and the rate of urbanization. And these indicators greatly affect the rate of spread of viral infections. In addition, having a drug that can overcome the virus in the root, a person does not need to deal with each symptom individually, that is, buy a bunch of other drugs (drops, sprays, tablets, suspensions, etc., etc.).
Scientists will continue their work, focusing on finding compounds that will be most effective against the maximum possible number of viruses. We can only wish them good luck and, until they have finished the job, fight the cold on their own.
Friday off-top:
Not only people suffer from viruses, but also computers, and sometimes animated characters. ( The second series is here.)
Thank you for your attention, remain curious, take care of your health and have a great weekend for everyone. :)
Not only people suffer from viruses, but also computers, and sometimes animated characters. ( The second series is here.)
Thank you for your attention, remain curious, take care of your health and have a great weekend for everyone. :)
Thank you for staying with us. Do you like our articles? Want to see more interesting materials? Support us by placing an order or recommending it to your friends, a 30% discount for Habr users on a unique analogue of entry-level servers that we invented for you: The whole truth about VPS (KVM) E5-2650 v4 (6 Cores) 10GB DDR4 240GB SSD 1Gbps from $ 20 or how to divide the server? (options are available with RAID1 and RAID10, up to 24 cores and up to 40GB DDR4).
Dell R730xd 2 times cheaper? Only we have 2 x Intel TetraDeca-Core Xeon 2x E5-2697v3 2.6GHz 14C 64GB DDR4 4x960GB SSD 1Gbps 100 TV from $ 199 in the Netherlands! Dell R420 - 2x E5-2430 2.2Ghz 6C 128GB DDR3 2x960GB SSD 1Gbps 100TB - from $ 99! Read aboutHow to build the infrastructure of the building. class using Dell R730xd E5-2650 v4 servers costing 9,000 euros for a penny?
