Inside the flame: a new method for investigating high-temperature reactive systems

In ancient Greek mythology, a special place is occupied by a character who zealously defended humanity from the cruelty and arbitrariness of the gods. Among other things, he gave us, people, fire and knowledge on how to preserve it. The name of this character is Prometheus. Zeus punished him in a very cruel and sophisticated manner - Prometheus was chained to a rock forever and ever, and the eagle every day pecked his liver, which completely regenerated, and the flour was repeated again. Not all physical or chemical phenomena receive their own mythology, but fire is another matter. Life-giving and at the same time destroying everything in its path, so simple and so mysterious. Today we will get acquainted with a work in which scientists demonstrated a new method for studying fire, which allows us to examine in more detail the molecular processes that occur in the tongues of flame. What tools and instruments were used by scientists, what new things could they learn about fire, and how can their work help humanity in the future? Answers invariably await us in the report of the research group. Go.
Study basis
Sometimes, looking at the flame, it seems that this is a living being with its thoughts and insidious plans. However, the mythical in the flame is as much as our imagination. In reality, fire is the same physicochemical process as crystallization of water, for example. Fire is an oxidation process that is accompanied by radiation in the visible range and the release of heat, that is, thermal energy. For the existence of fire, certain ingredients are needed: fuel, oxidizing agent and temperature. Imagine the most ordinary bonfire in the camp of tourists. Wood acts as a fuel, and oxygen, which is present in the air surrounding tourists, and, naturally, wood for a fire acts as an oxidizing agent. Without oxygen (i.e., an oxidizing agent in a broader sense), the combustion process is impossible. The third ingredient, temperature, determined by the properties of the previous two. There are a lot of variations of each of the constituent elements of fire, as well as their combinations, each of which has its own properties, characteristics and distinctive features. We have quite a lot of knowledge about the combustion process, but not all.
In the study being examined today, scientists decided to measure the temperature of the fire with various input variables: the temperature range of 1000-1800 K, pressure 2.0-2.9 at and 7.6-10.7 at, frequency 250 kHz. For this, a quantum cascade laser (QCL) with acousto-optical modulation (hereinafter AOM) with an average infrared range of the output signal from 1975 to 2260 cm -1 was used .
Scientists note that for the temporary measurements of non-intrusive particles in reactive systems, laser absorption spectrometry in the mid-infrared region is excellent. Comparison of the absorption forces of two target particles with different temperature dependences is already a two-line thermometry method. In this method, due to limitations in scanning speed and wavelength range, it is necessary to use several lasers at once for faster measurements. In addition, despite the sensitivity of measurements in low concentration media, narrow-band lasers are not suitable for systems with a high concentration of target particles.
Thus, a similar method cannot be used for temperature measurements in systems of energy-intensive materials, such as C 4 H 8 N 8O 8 (octogen) and C 3 H 6 N 6 O 6 (hexogen), since the target elements (H 2 O, CO, etc.) are produced in them in very high concentrations. Therefore, a new method is needed to study such systems, which scientists describe in their work.
Preparing the experimental setup
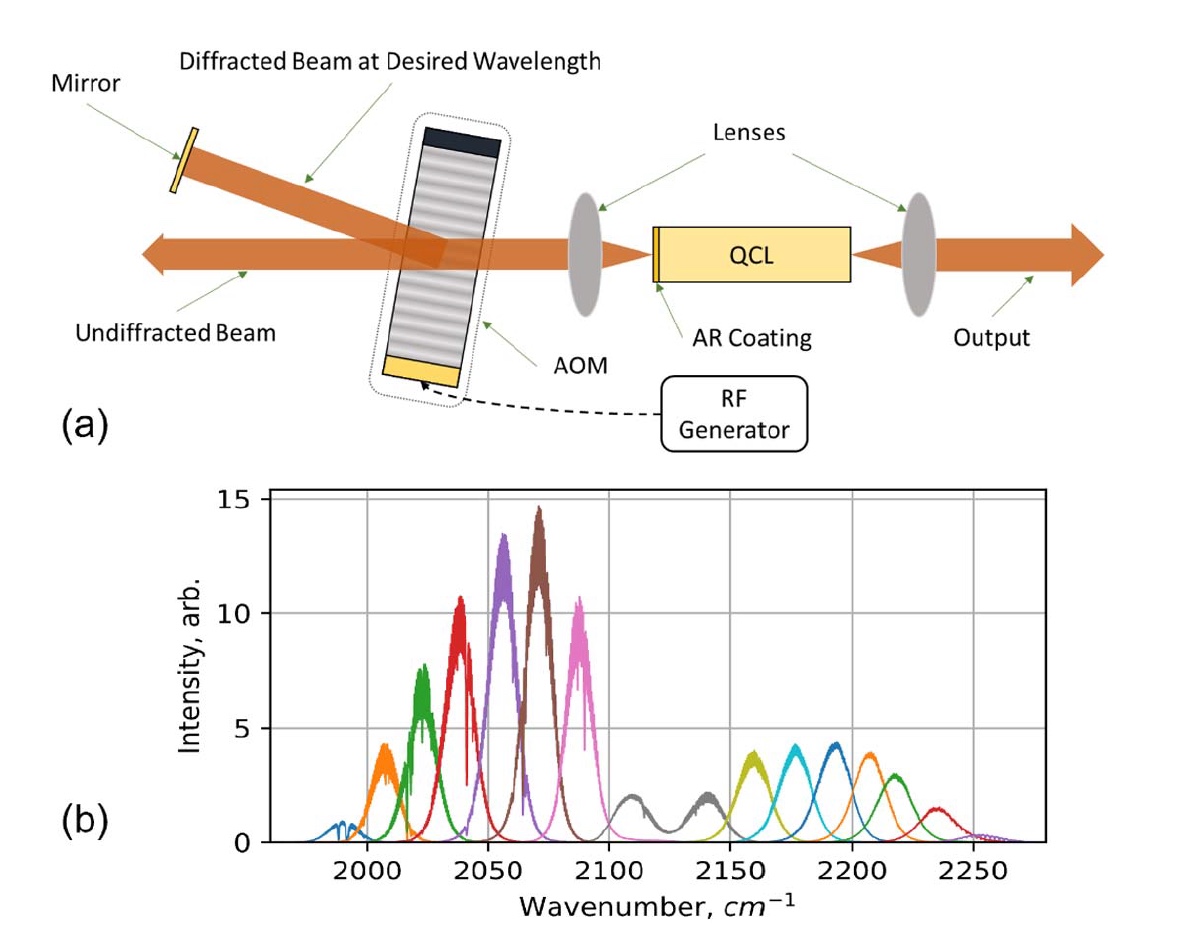
Image No. 1
Image 1a shows the experimental setup of a quantum cascade laser with acousto-optical modulation (AOM QCL):
- Mirror - a mirror;
- Diffracted Beam at Desired Wavelength - reflected beam at the desired wavelength;
- Undiffracted Beam - non-reflected beam;
- AOM - acousto-optical modulator;
- Lenses - lenses;
- QCL quantum cascade laser;
- AR Coating - antireflection layer;
- RF Generator - radio frequency generator;
- Output - output signal.
The spectral output signal AOM QCL was measured as a function of the input AOM RF using infrared radiation with Fourier transform with a spectral resolution of 0.2 cm -1 ( 1b ).
The half-width of the output signal depends on the operating conditions: pulse duration and QCL and AOM frequencies. In this experiment, the half-width index was approximately 12–15 cm –1 .
In the experiments, a stainless steel shock tube with an internal diameter of 14 cm was used, and polycarbonate diaphragms with a thickness of 0.18 and 0.76 mm were also used. Five piezoelectric pressure transducers located along the last 1.4 m of the shock tube were used to measure the impact velocity, which was linearly extrapolated to the end wall. The temperature and pressure in the reflected shock region (P5 and T5) were calculated using the initial temperature and pressure in this region and the extrapolated shock velocity using one-dimensional shock ratios taking into account chemically frozen, vibrationally balanced gases. The attenuation of the velocity was approximately 1.5% / m, and the error in T5 and P5 was less than 2%.

Image No. 2: experimental setup in conjunction with the test region of the shock tube.
Explanations for the image above:
- AOM QCL System - installation of a quantum cascade laser with acousto-optical modulation;
- RF Generator - radio frequency generator;
- Function Generator - Function Generator $
- Sync - synchronization;
- Pulsed Current Source - pulsed current source;
- Iris - aperture;
- I (transmitted signal intensity) Detector - sensor of intensity (I) of the transmitted signal
- Curved Mirror - a curved mirror;
- I0 Detector - reference beam intensity sensor;
- Beamsplitter - beam splitter;
- Mirror - a mirror;
- Endwall - end wall;
- ZnSe Windows - lenses from zinc selenide;
- Shock Tube - shock tube.
The beam from the AOM QCL was divided into reference and signal beams by means of a beam splitter made of calcium fluoride (CaF 2 ). The intensity of the reference beam was measured using a thermoelectric cooled photoelectric sensor. As we can see from the diagram, in front of the tube, the beam passes through a lens of zinc selenide 3 mm thick and 12.7 mm in diameter. Both lenses were located at a distance of 2 cm from the end wall and were aimed at each other. Having passed the second lens, the beam is directed to the intensity sensor of the transmitted signal through a curved mirror.
The setup laser operated in a pulsed mode with a repetition rate of 500 kHz and a pulse duration of 100 ns. AOM was used to alternate pulses between spectral bands of 2030 cm -1 and 2080 cm -1by modulating the radio frequency driver with a meander * with a period of 250 kHz, which was synchronized with the laser pulse driver.
The meander * is a periodic signal of a rectangular shape.Such precise spectral bands were specially selected to provide high temperature sensitivity in the studied temperature range with relatively low sensitivity to the mole fraction and CO pressure. In addition, for more spectral stability, AOM and QCL were temperature controlled.
Experiment Results
And now we will pass directly to the results of the installation.
During the experiments, the measured temperature varied from 1000 to 1800 K, and two pressure options were used: low - 2.0-2.9 at. And high - 7.6-10.7 at. The analyzed mixture consisted of CO diluted in helium (He) and argon (Ar). A mixture of 10% CO, 25% He and 65% Ar was used at low pressure, and 3% CO, 15% He and 82% Ar at high pressure. To ensure the homogeneity (homogeneity) of the samples, the mixing process proceeded for 8 hours.

Image No. 3
To calculate the expected spectrum of the signal beam for each of the two bands along the wavelength, we used a combination of the simulated absorption spectrum and the measured output spectrum AOM QCL. In this case, the Behr - Lambert monochromatic law was taken into account for each individual wavelength ( 3a ).
The absorption for two bands was simulated for each combination of T5, P5 and molar fraction taking into account the 14 cm path (shock tube length) for: temperature range 600-2600 K in increments of 50 K, range of molar fractions of CO from 1% to 50% in increments 1% and a pressure range of 0.001-13.0 bar in increments of 1 bar.
As seen in image 3b, the temperature strongly affects the absorption coefficient, but slightly on the mole fraction and pressure. The temperature and mole fraction were calculated using an iterative method, i.e. these indicators were first determined separately from each other and used to calculate the theoretically expected absorption values for the two output bands at the experimentally measured pressure (P5). After that, the temperature was changed by comparing the theoretical and measured absorption coefficients. The molar fraction of CO was changed using the difference between the measured and theoretical values of the absorption band of 2080 cm -1 .
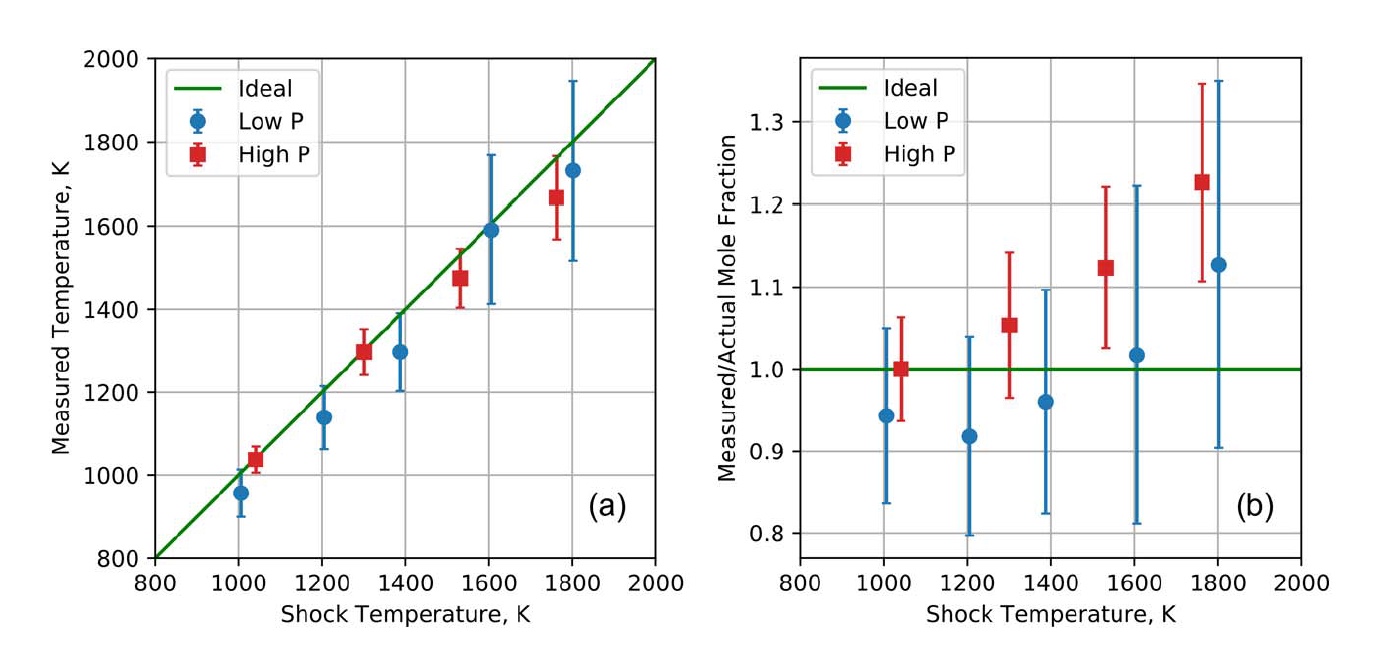
Image No. 4
On image 4awe can see a comparison of measured and known temperatures in shock tube experiments when taking into account impacts of both low and high pressure. As we can see, the temperatures that were obtained using the AOM QCL system are almost perfectly consistent with the shock temperatures over the entire range of 1000–1800 K and at both pressure ranges. The adjacent graph ( 4b ) shows the ratio of the measured and actual molar fraction of CO. In the case of this indicator, excellent agreement is also observed between the initially known data and those obtained by measurements with the AOM QCL experimental system.
Researchers Findings
Scientists have found that the temperature change is independent of the displacements of the laser beam arising from the particles of the diaphragm. This conclusion is justified by the fact that this bias affects both spectral components of the output signal due to variable pulses passing through the same beam. As a result, the offset is compensated.
If we take into account the immunity of temperature measurements to background thermal emissions and the above-described shift of the laser beam, then the developed method is excellent for studying gas-phase reactions of energy materials (for example, octogen and hexogen), in which CO is generated, and hot particles and pressure waves can cause thermal emissions and beam displacement.
Also, given the bandwidth of the AOM QCL at 12-15 cm -1, many absorption characteristics of one component of the studied medium can be analyzed simultaneously. Narrow-band lasers have an increased sensitivity, but are limited in the concentration range where they can be used, due to saturation.
HITEMP spectrum modeling takes into account only CO. Accordingly, the use of the AOM QCL system on structures, when the component components of the mixture can be different, requires further improvement of the system to increase its accuracy.
For a more detailed acquaintance with the nuances of the study, I strongly recommend that you look into the report of scientists .
Epilogue
This experimental study is a demonstration of a new tool in the study of temperature and component concentration inside high-temperature reactive systems. Scientists using this tool were able to successfully study mixtures with 3% and 10% CO in the temperature range of 1000 ... 1800 K at a pressure of 2.0-2.9 at and 7.6-10.7 at.
The AOM QCL system, according to the developers themselves, is quite flexible and allows you to configure it for various studied environments in a wide temperature range. In addition, the system can measure several components of the medium at once by measuring their absorption characteristics.
Fire is not just a stove in a village house, a fireplace in a mansion or a candle on a cake. Fire is a complex physical and chemical process, the understanding of which gives a person more tools to control his creative power and fight against his destructive power.
I do not exaggerate saying that we were all shocked by the fire that occurred in the Notre Dame Cathedral. So many centuries of scientific research, discoveries and breakthroughs, but we could not save one of the greatest and most beautiful pearls of architecture from rebellious fire. This loss once again reminded us that man is so great, and we still have to learn a lot about the world around us in order to completely protect ourselves from the troubles that he can present to us. The only destructive force with which we most likely will never be able to control this is ourselves.
Thank you for your attention, remain curious, remember the rules of fire safety and a good working week, guys.
Thank you for staying with us. Do you like our articles? Want to see more interesting materials? Support us by placing an order or recommending it to your friends, a 30% discount for Habr users on a unique analogue of entry-level servers that we invented for you: The whole truth about VPS (KVM) E5-2650 v4 (6 Cores) 10GB DDR4 240GB SSD 1Gbps from $ 20 or how to divide the server? (options are available with RAID1 and RAID10, up to 24 cores and up to 40GB DDR4).
VPS (KVM) E5-2650 v4 (6 Cores) 10GB DDR4 240GB SSD 1Gbps until the summer for free when paying for a period of six months, you can order here .
Dell R730xd 2 times cheaper? Only we have 2 x Intel Dodeca-Core Xeon E5-2650v4 128GB DDR4 6x480GB SSD 1Gbps 100 TV from $ 249 in the Netherlands and the USA! Read about How to Build Infrastructure Bldg. class using Dell R730xd E5-2650 v4 servers costing 9,000 euros for a penny?
