About simple things, complicated. "Sleeping steel." How to grease rusted bolts or Not WD-40 with a single ...
- Tutorial
Dedicated to all desperate and hopeless ... loosen a rusted nut!
Spring definitely came to the streets of our cities. It became warmer and people with joy began to give preference to their real iron friends, at least temporarily forgetting about tablets and smartphones. Cyclists, motorcyclists and many more lovers of seasonal vehicles got their go and suddenly found that something was rusted, something could not be unscrewed, etc. etc. I admit, I am one of those who discovered :) And so that the work on combating corrosion would not be in vain, I decided to fill out the accumulated material in a habr article.
The information will definitely be useful to absolutely everyone who has at least once had to deal with rusty parts, not only car enthusiasts and homemakers, but also restorers of equipment, those who are going to paint rusty posts in the country / suffer from rusty stains on the sink and just want to get to the bottom of the process rusting and find methods to effectively combat this scourge. Today we are talking about how to wake up the “asleep steel”.
Well, traditionally - do not forget to bookmark,% USERNAME%, come in handy! :)

We live in a world of iron and its alloys. And where there is iron, there will certainly be its oxides in the form of rust. Any iron element will rust in the open air, the only question is how fast. When exposed to water, oxygen, aggressive gases contained in air, ferrous metals easily transform into chemically resistant forms of their compounds. This natural process of the transition of metals to oxides, hydroxides and salts begins with the surface, so the unprotected surface of ferrous metals is always covered with a film of corrosion products. The thickness of these films depends on the conditions of formation and ranges from fractions of a micrometer to several millimeters. The corrosion process develops over time, even under favorable storage conditions, since many salts are hygroscopic,
In fact, metal rusting is simply the oxidation of iron by atmospheric oxygen, in which water acts as a “catalyst." All this is described by three main reactions:
O 2 + 4e - + 2H 2 O → 4OH -
Fe → Fe 2+ + 2e -
4Fe 2+ + O 2 → 4Fe 3+ + 2O 2 -
Iron, being a fairly active metal, gives off electrons and oxidized, water accepts these electrons and alkalizes the reaction medium with OH - ions. Ferrous ions in combination with OH -precipitate in an insoluble precipitate of iron (II) hydroxide, which gradually in the presence of the same oxygen begins to form various combinations of oxides / hydroxides, including due to the processes of stepwise dehydration.
Fe 2+ + 2H 2 O ⇌ Fe (OH) 2 + 2H +
Fe 3+ + 3H 2 O ⇌ Fe (OH) 3 + 3H +
Fe (OH) 2 ⇌ FeO + H 2 O
Fe (OH) 3 ⇌ FeO (OH) + H 2 O 2
FeO (OH) ⇌ Fe 2 O 3 + H 2 O
The composition of rust, respectively, slowly changes over time, depending on the conditions of the surrounding atmosphere (excess / lack of oxygen and water)
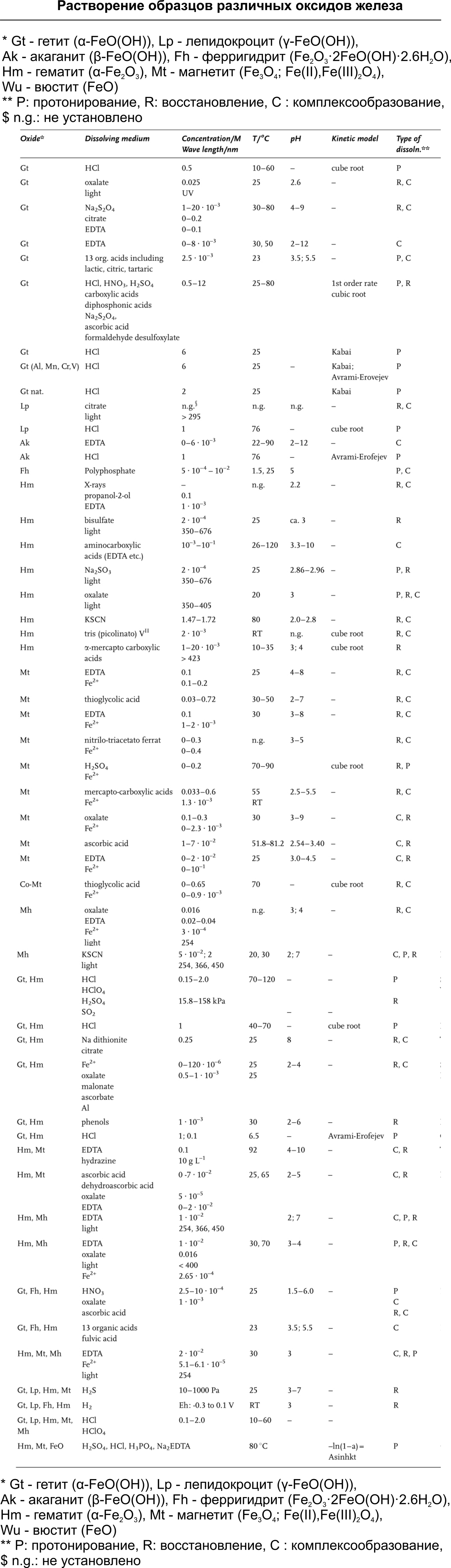
As already mentioned, the composition of the formed rust may vary depending on the type of steel, the presence of electrolytes, the aggressiveness of the impact and its duration. In general, it is believed that there are 16 iron oxides having a different crystalline structure, chemical composition and valence state of iron, which theoretically can be found in rust. In our area, most often, rust formed at room temperature may contain goethite (α-FeO (OH)), acaganite (β-FeO (OH)), lepidocrocyte (γ-FeO (OH)) and magnetite (Fe 3 O 4 ; Fe (II), Fe (III) 2 O 4) I recommend remembering these names, they are still useful. Most researchers agree that the main crystalline component of rust is γ-FeO (OH), which, when heated, transforms into γ-Fe 2 O 3 . If a part or product rusts for a long time in a humid atmosphere, small amounts of Fe 3 O 4 (often of non-stoichiometric composition) can be detected in rust . Rust samples in distilled water are rust from crystalline α-FeO (OH), γ-FeO (OH) and Fe 3 O 4 . If the metal undergoes rust in the salt spray chamber , then the main crystalline component of rust is γ-FeO (OH) with a lamellar and porous structure.
I would also like to recall that salts, in particular chloride ions, act as a kind of electrochemical catalyst that accelerates corrosion (our winter roads and vehicle bottoms will not let you lie) and contribute to the formation of γ-FeO (OH). There are studies in which the authors compare the rust taken for analysis in various places (coastal, continental, etc.). The rust formed in the coastal areas was mainly in the form of large flakes; in areas with high moisture and chlorides in the soils, leaf-shaped rust was formed, and powdery and fine-grained rust was the lot of the central and northern territories. Layered rust samples contained γ-Fe 2 O 3 · H 2 O on the contact surface with air and Fe 3 O4 on the metal contact surface, α-FeOOH and δ-FeOOH were found in the intermediate layers and in flakes.
Why am I telling all this, and then that I need to know the enemy in person. The more accurately determine the type of rust - the more efficient it can be dissolved.
It is well known that depending on environmental conditions, multi-colored rust may form: red rust (hydrated oxide Fe 2 O 3 · H 2 O is formed at high levels of oxygen and water vapor, most often it is uniform atmospheric corrosion in very aggressive environments.), yellow rust (so-called solvated rust, soluble FeO (OH) · H 2O образуется в условиях высокой влажности, чаще всего, если металл находился в луже/стоячей воде), бурая ржавчина (сухой оксид Fe2O3, который образуется при высоком содержании кислорода и низкой влажности, чаще представляет собой локализованную ржавчину, которая проявляется в виде неоднородных пятен или только в определенных областях (загрязнения и дефекты на поверхности металла) и черная ржавчина (оксид Fe3O4 который образуется в среде с низким содержанием кислорода и низкой влажности, является устойчивым видом ржавчины, похожим на слой покрытия, возникающего при оксидировании металла).

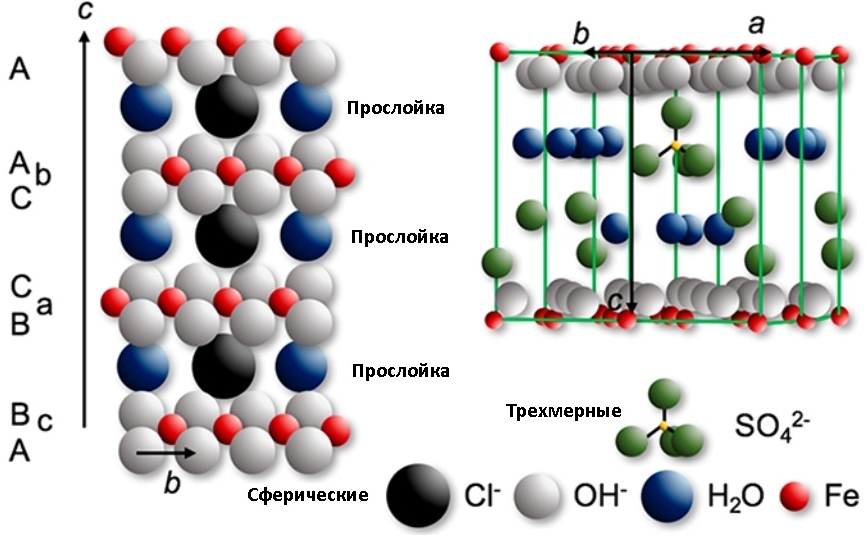
If ions of various inorganic acids come into play (carbonates, sulfates, chlorides already mentioned, as well as bromides, fluorides, iodides, nitrates and selenates), so-called green rust

Green rust is the common name for various crystalline green compounds containing iron cations and the anions mentioned above. This beauty (?) Is formed on iron / cast iron / steel surfaces exposed to water containing chloride, sulfate, carbonate or bicarbonate ions during alternating aerobic / anaerobic conditions. For example, in ships, bridges, etc. A general view of the crystal structure of green rust is shown in the picture. In principle, we can assume that the structure of the usual “shades of red” rust is in many ways similar to green, but without acidic anions.
Although rusting is generally a negative aspect, there are examples where it also serves the person. An example is the bluing of weapon trunks (including processing with the so-called “rust varnish”) and homemade knife blades.
A thin oxide film of “black rust” (described above) is formed on the metal, protecting the metal from further oxidation. This process is also called oxidation:
As an example, we can mention the iron column (aka Kutubova column ) in Delhi - a cylinder seven meters high and weighing six and a half tons, which is part of the Qutb Minar architectural ensemble, located about 20 kilometers south of Old Delhi. The column gained fame by the fact that for 1600 years of its existence it virtually avoided corrosion.

What they didn’t expect about this column, from “made of meteorite iron” to “gift of Shiva”. But, traditionally, the answer was simple - “high temperature and dry air”will save the father of Russian democracy. A thin oxide film protects the metal from rust if it is exposed to only fresh water or dry air.
If we briefly introduce the mechanism of thread rusting, it turns out that it practically does not differ from metal rusting on the surface. Initially, water enters the inter-threaded channels (between the nut and the bolt), which, in combination with air oxygen and iron electrons, starts the processes described by the formulas above. As a result of this process, iron oxides and hydroxides begin to form, which, depending on the conditions, undergo hydration / dehydration cycles and form a monolithic porous structure. It can be said that rusting inside a thread differs from rusting an open metal in that a lack of oxygen can be observed inside the thread and something similar to black rust will form (Fe 3 O 4 ).
Probably the closest phenomenon to “souring” is rusting of reinforced concrete. The same conditions lack oxygen. Under such conditions, the volume of loose oxides formed as a result of oxidation is much larger than the volume of the reacted metal. Oxides completely fill all pores and leaks (threads or protrusions near the reinforcement), acting as a kind of proppant or sealant.
As a result of the described process, slowly but inexorably true, rust presses everything in contact with it and destroys concrete, blocks the thread, etc. There is even such a thing as “rust packing” or “pack rust”, which in translation into the great and mighty means “packet rust”. It is observed in those cases when the volume of the formed oxides with its wedging pressure squeezes out metal parts adjacent to the places of corrosion. The most famous example of the effects of batch rust can be the collapse of the bridge over the River Myanus in the United States in 1983 with many casualties.

A special investigation found that corrosion resulting from rainwater entering the metal structures of the bridge due to impaired drainage technology gradually leaked to the iron mounts. The fasteners gradually rusted and pushed one corner of the road slab from the support in millimeters. When the distance became sufficient for destruction, one passing car served as a trigger. Since then, a new term has appeared in bridge construction and is actively used when signs of rust accumulation between steel plates and bridge joints are observed during inspection of the bridge
I hope the mechanics of the process are approximately clear. It is needed in order to evaluate the existing methods of dealing with threaded (almost "batch") rust.
In the simplest way, in order to unscrew the bolt you need to do two things
This can be done by various methods:
destruction_method 1 - Mechanical
In fact, grandfather. Because for a long time, in the absence of other possibilities, it is customary to knock on a rusted thread in the hope of destroying monolithic plates and chains of formed oxides with vibration. As soon as the bolt is removed, the oxides (and they are fragile enough) will continue to rub themselves into dust. The method is not particularly effective and, in addition, requires a specialist who "feels a hammer", so as not to inadvertently twist or rivet a bolt tightly.
A good option is to use electric or pneumatic impact wrenches (+ there are also screwdrivers, added redbeardster in the comments ), like the one in the picture:

Although in this case, as well as with an ordinary hammer, the main thing is not to overdo it and not break the bolt. It is better to install a correctly selected head from the side of the nut, and at the same time support the bolt with an additional wrench.
In case there is no need to save the fasteners (for example, in the case of antique cars), you can simply cut the nut with an angle grinder (grinder), and drill the bolt. But the labor costs for such a procedure are such that I would advise using this method in the last turn, having tested all the possibilities described in the article.
Addition: I remembered about the grinder , but about the wrenches (they are nut cutters and nut cutters) - no. Thanks to the p_fox reader who reminded me of such a thing.

destruction_method 2 - Thermal
This method is based on the understanding that all bodies expand when heated, and when cooled, they contract. Heating the nut (or the object into which the bolt is screwed) will lead to the formation of micro-fractures in the oxide monolith located along the thread. The alternation of heating / cooling cycles is very likely to lead to crushing of rust flakes and the formation of "vacuum". And as soon as holes are formed inside the layer, the bolt will most likely turn around. In addition to mechanical crushing of the oxide layers due to the expansion of the metal, dehydration of the rust components themselves takes place. For example, annealing at 350 ° C turns rust mainly into maghemite (γ-Fe 2 O 3), magnetite forms at 550 ° C, and at 750 ° C the rust turns into a mixture of magnetite, wustite (FeO) and metallic iron (Fe).

Previously, this method was available only to owners of acetylene or propane burners, but with the advent of aliexpress today, almost everyone can buy a compact burner for a “dichlorvos” cylinder and calcine rusted bolts and nuts for their own pleasure.

Actively thermal removal of rust is also used in the restoration of iron products. True, this is not just annealing, but the high-temperature reduction of oxides into elemental iron. This is done either by heating the rusted products in a carbon monoxide medium (aka CO, aka carbon monoxide) under a layer of charcoal with limited air access and a temperature of 800 ° C. Hydrogen can also be used as a reducing agent, especially if there is access to tube furnaces with temperature controlled along the length of the furnace. Ammonia is fed into the reaction part of the furnace, which decomposes on the catalyst at 400-600 ° C into nitrogen and hydrogen. Hydrogen reduces oxides to “sponge iron”, which further requires additional treatment with protective agents, such as molten paraffin.
Addition:In the same section I will introduce the laser rust cleaning feature mentioned by the Alexus819 reader , which is perfectly applicable for smooth surfaces (see clickable video).

destruction_method 3 - Chemical The
chemical method for the destruction of threaded rust is based on the fact that chemical components entering the pores and capillaries of the oxide layer can interact with it, either converting rust into a soluble compound or reducing it to metallic iron. Both relieve the proppant pressure inside the thread and allow the nut to be rotated due to the formation of additional pores or areas of reduced density. In general, the mechanisms of influence of the chemical method can be divided into three directions: protonation , complexation , reduction. No wonder I quoted the names of the most common “minerals” forming rust at the beginning of this article. I did this so that an inquisitive reader could pick up a suitable reagent, hidden in a table under the spoiler.
And now, a little detail about each of the mechanisms with examples.
Protonation
As a result of protonation, reagents capable of becoming a proton donor (H + ) react with rust . Most often, mineral inorganic acids are used for this purpose.
Traditionally, solutions of mineral acids are used to clean the surface of iron from corrosion products. The most active is a solution containing 35% orthophosphoric and 5-10% hydrochloric acid. Solutions of acids - sulfuric, hydrochloric - allow you to quickly remove corrosion products, but always cause some metal pickling. To prevent this, corrosion inhibitors are introduced into acid solutions. So, in 1 M (I recall, just in case, that a solution of a concentration of 1 M contains 1 mol of a substance per liter of solution) sulfuric acid solution, it is advisable to add thiosemicarbazide, thiourea, urotropin, triphenylphosphine, benzotriazole (good results are achieved when iron is treated with a 1 M sulfuric acid solution containing 0.1-0.5% thiourea or 0.5-1.0% benzotriazole); in 1 M hydrochloric acid solution - urotropine and triphenylphosphine.
Note: Triphenylphosphine is an organic compound with the formula P (C 6 H 5 ) 3 , or simply Ph 3 P. It is a derivative of phosphine. It has the appearance of white crystals. Relatively stable when stored in air.
Triphenylphosphine is also interesting because its derivative, triphenylphosphine oxide, is widely used in microelectronics, and by its smell, Labrador dogs in the USA are taught to find various electronic memory devices. Under the spoiler excerpt from Science and Life No. 10/2018

Since we started talking about inhibitors, in addition to the ones mentioned above (and available in the ASTM tables below), we can also mention various amines that are found in branded “rust solvents”. Monoethylamine, diethylamine, triethylamine, etc. are actively used. (which is at hand).
Complexation
Complexation - the process of the so-called coordination compounds. They are neutral molecules or ions resulting from the addition of neutral molecules or other ions called ligands to an ion or atom called a complexing agent. Most often, ligands act as bulk organic molecules.
Most of the organic acids used in dissolving rust work precisely according to the mechanism of complex formation with Fe (III) ions. The best efficacy is shown by formic, citric and oxalic acid (as well as their salts), ethylenediaminetetraacetic acid (EDTA) and its salt Trilon-B, which is actively used in all kinds of Calgons to remove scale. When using solutions of organic acids, corrosion inhibitors (the same urotropin) can be added to their composition, since acids, albeit slowly, but still sometimes cause metal pickling. I would like to note that organic acids work better in the presence of small amounts of mineral acids (pH adjuster) and when heated (see the picture under the spoiler).
Recovery
Well, and finally, the third mechanism by which structural bonds between iron atoms in iron oxides can be weakened and destroyed is the reduction of structural Fe (III) to Fe (II) (or even Fe 0, if you're lucky). Its essence lies in the processes of electron transfer and the related adsorption of an electron donor, cathodic polarization of the electrode, electron transfer from unstable surface complexes to the Fe (III) surface, and much more matan. More important, in my opinion, is that a huge number of compounds are used as reducing agents - sodium dithionite (bisulfite), thioglycolic acid (it is also mercaptoacetic, widely used for perming and dyeing hair) acid, thiocyanate, hydrazine, ascorbic acid, hydroquinone, hydrogen sulfide fructose, sucrose. And even such amazing things as sulfonic acids from the soil:
Under appropriate conditions, reductive dissolution can also be carried out photochemically.
The most popular reducing agents are hydrogen peroxide, a 3-5% aqueous solution of NaOH and sodium sulfite Na 2 SO 3 , the sodium bisulfite already mentioned above, ascorbic acid. Often, zinc powder in a 15% NaOH solution (which, by the way, when applied to smooth rusty surfaces, is additionally thickened with polyvinyl alcohol) is used as a reducing solvent for rust
. Comparison of the effectiveness of various methods for dissolving rust
Often, to dissolve rust, not individual reagents are used, but their combinations, where each substance implements its own mechanism for dissolving iron oxides. An example is the same zinc + NaOH in which Trilon B is added to speed up the process, thereby regulating the cleaning ability of the mixture. Below the spoiler is a picture with which you can compare the dissolution rate of rust using various mechanisms.
Below is a diagram that shows that rust dissolution methods using combinations of different (heterogeneous) “oxide layer destroyers” are most effective.
Well, to summarize, I will give a methodological recommendation, taken from the American ASTM standard on the removal of corrosion products from metal. The Americans themselves, as I understand it, most likely focused on the processing of materials on the plane, but everything below can easily be applied to threaded joints.
Note from a colloidal chemist : it must be understood that in the case of a thread (as opposed to rust on the surface), in order to destroy all the oxides along the thread, the above compounds must reach them (the reaction proceeds only at the place of direct contact between the reactant and the oxide). And to do this is quite difficult, since all penetration paths are tightly clogged with rust that has not yet had time to react. Therefore, in addition to the dissolution efficiency, the wetting effect of the reagent (the ability to penetrate into pores, microcracks, and capillaries) should be taken into account. The following section is devoted to these issues.
As I wrote above, after chemical, thermal or mechanical destruction of the oxide layers of rust, it is necessary to reduce the friction between them. It is logical that this can be done using grease. Most “folk” remedies, such as various oils, kerosene, gasoline, acetone, do not change the state of rust inside the “sour” bolt, but they can help themselves in turning the nut together with the fragments of the oxide layer after the initial treatment to destroy the porous frame.
Important!Pouring any hydrocarbons onto the “acidified” thread makes sense only if the path along the thread is not completely clogged with oxides, in which case the solvent penetrates / adsorbes into them and only then acts as a lubricant. Those. it is necessary to moisten with all kinds of kerosene either the bolts that are not very rusted or when the bolt has already been touched and pores have formed in the oxides. Therefore, given the foregoing (for example, the paragraph thermal destruction) it would be logical to apply “kerosene” to the rusted thread, and then simultaneously with tapping the bolt head with a hammer or other percussion instrument, try to move the nut from its place. For cast-iron and steel water pipes with a thread, experts recommend heating the rusted places, applying paraffin from a candle, heating them again until the melted hydrocarbon is absorbed and slipping along the threads and only then try to unwind.
A note about the WD-40.Many probably heard about this thing, the "triumph of American petrochemicals." I, unlike some friends who cannot imagine a car without a WD-40 bubble in the glove compartment, do not feel much reverence for this mixture of hydrocarbons. For those who are not in the know, this is a solution that was developed in the 60s of the last century to protect the corps of American missiles from rust and corrosion. Well, then, as usual, ordinary Americans appreciated the benefits of this incomprehensible liquid. "WD-40" is an abbreviation for the term "water displacement, 40th formula", i.e. for the 40th time they did something. Composition WD-40 has never been patented, in order to preserve trade secrets. Therefore, it is still not really clear what is in the original development. Every year it’s becoming more and more difficult to find out (because according to the stories of knowledgeable people>brought this "kerosene" onto a gas chromatograph + mass spectrometer and found that it contains: mineral oil, decane, nonane, undecane, tridecane, tetradecane, cyclohexane, dimethylnaphthalene and carbon dioxide to create the necessary pressure in the cylinder. MSDS (safety data sheet for the US market) gives the following information: 50% - aliphatic hydrocarbons, <25% - mineral oil, 12-18% aliphatic hydrocarbons with low vapor pressure to reduce the viscosity of the solution (easily volatile diluent), 2-3% carbon dioxide, <10% inert ingredients.
The solution mentioned above is often praised for its incredible permeability (or permeability, I don’t remember exactly how WD fans say it). If you look from the point of view of colloid chemistry, it turns out that these people most likely mean the phenomenonwetting . Briefly, it depends on the forces of intermolecular interaction and consists in the following: if the forces of interaction between molecules of a liquid and a solid are greater than between molecules of a liquid, then the liquid spreads over the surface of the solid, i.e. it wets and vice versa, if the interaction forces between the liquid molecules are greater than between the liquid and solid molecules, then the liquid collects in a drop and does not wet the surface of the liquid. This is directly related to such a thing as surface tension .
Tatko napaminae : so that the son does not forget to mention such a thing as “red tormozuha”, it’s also brake fluid BSK (like “butyl-alcohol-castor oil” in a 1: 1 ratio).
While someone was using the WD-40 from the glove compartment, if I was disassembling a motor and suddenly there was a tightly rusted fastener, the following happened. Tatko silently looked at this matter, then silently went to the garage and brought a syringe with a red liquid of pungent odor. The liquid was applied, aged 30 minutes and ... And indeed, in most cases it worked and the nut could be unscrewed. In fairness, I note that all the fasteners for which BSK was used were in motors, where there was always a certain amount of lubricant. To be honest, it is very likely that my father still has this brake fluid in the garage, which is stored specifically in case of rusted fasteners. It’s hard to find such an option on sale now, because manufacturers abandoned butanol in favor of various polyglycols and their esters, which penetrate the thread capillaries much worse. Perhaps this is due to the fact that polyglycols are cheaper, and maybe because they are safer.
Addition: @ Alexey Shukaev clarifies that the transition from butanol to polyglycols is associated with a difference in boiling point. "The transition to gaseous => compressibility => the hydraulics stops working" - so I had to refuse ...
In my memory there were examples of cases when people, tired of finding money for factory booze, drank red brake fluid. Butanol, after all from the same series of alcohols as ethanol, even though it has the highest toxicity among simple alcohols (LD 50is 2290-4360 mg / kg). Most fusel oils in alcohol production by distillation are butanol. It is he who gives the furious, incomparable hangover. But this is when "digested." And castor oil, it is known for its therapeutic laxative effect. In general, a multifunctional product was produced in the USSR ...
Note : in the modern world, tired of the unsuccessful search for a mixture of castor oil and butanol, its analogue (of the same color) is used: transmission (red) oil from an automatic transmission (ATF) and acetone in a ratio of 1 :1. Color similar, efficiency too.
And from all this it follows that if a liquid (some solvent) has a surface tension less than water, then it will be better to moisten the rust and penetrate into the pores and capillaries faster than an aqueous solution of any acid. It will be better to penetrate, but it will not be able to destroy or weaken the bonds between the oxide layers. The following observations are emerging:
1) WD-40, and all kinds of “liquid keys” (English liquid wrench) - are ordinary hydrocarbons and components close to them, which have low surface tension and are able to wet porous oxides well and penetrate their capillaries.

These, as a rule, oil products, are perfectly absorbed by thread threads and provide lubrication. Only lubrication, because all components themselves are inert and do not have any noticeable effect on rust. From the word in general. Therefore, it is best to use them after / in conjunction with the methods of destruction of oxide layers described in the article. The sad thing is that even RUST REMOVERs recognized by rust would not corrode and penetrate perfectly.
2) Any PB-Blaster, Rust buster and Rust dissolver declared as a solvent for rust, really dissolve rust. As a rule, they also contain a component that reduces surface tension and gives a light lubricating effect. But this effect is deeply secondary. Under the spoiler a couple of famous examples:
As you can see, all the same vigorous inorganic acids and alcohols are used to give them the necessary mobility and reduce surface tension. + In some cases, corrosion inhibitors. Those. theoretically, everyone can experimentally make their own rust solvent by mixing their favorite (= available) inorganic acid with alcohol (available).
Important : all the chemical methods of rust destruction and transformation described in the article can be used not only to dissolve “sour” threads on bolts, but also for anticorrosion treatment of metal (iron / steel / cast iron) of any shape, as well as when removing rusty stains on plumbing etc.
Well, for a snack I would like to offer such a fact. Recently, in connection with the desire of manufacturers of chemical compounds to comply with the concepts of green chemistry , constantly searching for new, more environmentally friendly and biodegradable components. All kinds of solvents and rust converters did not stand aside. The latest trend is the use of organic compounds of a phenolic nature, tannins, as a transforming component (instead of the usual phosphoric acid and iron phosphate, for example). The tannin action of these substances turns the reddish iron oxides into a bluish-black stable tannate. Here you have a place where you can attach astringent persimmonwith her tannins :). And in general, theoretically, it’s quite an option to treat the rusty bottom of your favorite car, instead of toxic inorganic acids, with a strong infusion of green tea ...
This is the end of the rust story, subscribe to my Facebook / VKontakte notes to know more and be in the subject of the latest research!

Spring definitely came to the streets of our cities. It became warmer and people with joy began to give preference to their real iron friends, at least temporarily forgetting about tablets and smartphones. Cyclists, motorcyclists and many more lovers of seasonal vehicles got their go and suddenly found that something was rusted, something could not be unscrewed, etc. etc. I admit, I am one of those who discovered :) And so that the work on combating corrosion would not be in vain, I decided to fill out the accumulated material in a habr article.
The information will definitely be useful to absolutely everyone who has at least once had to deal with rusty parts, not only car enthusiasts and homemakers, but also restorers of equipment, those who are going to paint rusty posts in the country / suffer from rusty stains on the sink and just want to get to the bottom of the process rusting and find methods to effectively combat this scourge. Today we are talking about how to wake up the “asleep steel”.
Well, traditionally - do not forget to bookmark,% USERNAME%, come in handy! :)

Chemical background
We live in a world of iron and its alloys. And where there is iron, there will certainly be its oxides in the form of rust. Any iron element will rust in the open air, the only question is how fast. When exposed to water, oxygen, aggressive gases contained in air, ferrous metals easily transform into chemically resistant forms of their compounds. This natural process of the transition of metals to oxides, hydroxides and salts begins with the surface, so the unprotected surface of ferrous metals is always covered with a film of corrosion products. The thickness of these films depends on the conditions of formation and ranges from fractions of a micrometer to several millimeters. The corrosion process develops over time, even under favorable storage conditions, since many salts are hygroscopic,
In fact, metal rusting is simply the oxidation of iron by atmospheric oxygen, in which water acts as a “catalyst." All this is described by three main reactions:
O 2 + 4e - + 2H 2 O → 4OH -
Fe → Fe 2+ + 2e -
4Fe 2+ + O 2 → 4Fe 3+ + 2O 2 -
Iron, being a fairly active metal, gives off electrons and oxidized, water accepts these electrons and alkalizes the reaction medium with OH - ions. Ferrous ions in combination with OH -precipitate in an insoluble precipitate of iron (II) hydroxide, which gradually in the presence of the same oxygen begins to form various combinations of oxides / hydroxides, including due to the processes of stepwise dehydration.
Fe 2+ + 2H 2 O ⇌ Fe (OH) 2 + 2H +
Fe 3+ + 3H 2 O ⇌ Fe (OH) 3 + 3H +
Fe (OH) 2 ⇌ FeO + H 2 O
Fe (OH) 3 ⇌ FeO (OH) + H 2 O 2
FeO (OH) ⇌ Fe 2 O 3 + H 2 O
The composition of rust, respectively, slowly changes over time, depending on the conditions of the surrounding atmosphere (excess / lack of oxygen and water)
Rust mechanism in one picture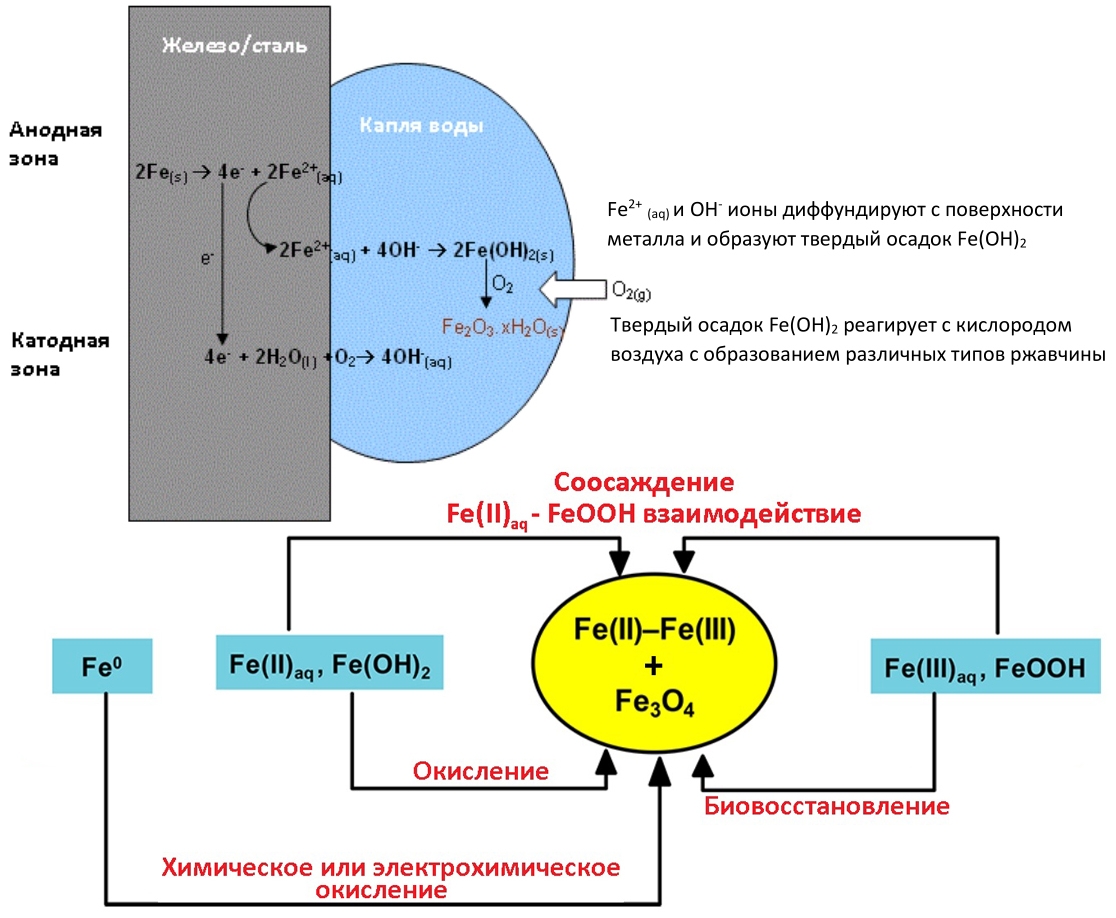

As already mentioned, the composition of the formed rust may vary depending on the type of steel, the presence of electrolytes, the aggressiveness of the impact and its duration. In general, it is believed that there are 16 iron oxides having a different crystalline structure, chemical composition and valence state of iron, which theoretically can be found in rust. In our area, most often, rust formed at room temperature may contain goethite (α-FeO (OH)), acaganite (β-FeO (OH)), lepidocrocyte (γ-FeO (OH)) and magnetite (Fe 3 O 4 ; Fe (II), Fe (III) 2 O 4) I recommend remembering these names, they are still useful. Most researchers agree that the main crystalline component of rust is γ-FeO (OH), which, when heated, transforms into γ-Fe 2 O 3 . If a part or product rusts for a long time in a humid atmosphere, small amounts of Fe 3 O 4 (often of non-stoichiometric composition) can be detected in rust . Rust samples in distilled water are rust from crystalline α-FeO (OH), γ-FeO (OH) and Fe 3 O 4 . If the metal undergoes rust in the salt spray chamber , then the main crystalline component of rust is γ-FeO (OH) with a lamellar and porous structure.
I would also like to recall that salts, in particular chloride ions, act as a kind of electrochemical catalyst that accelerates corrosion (our winter roads and vehicle bottoms will not let you lie) and contribute to the formation of γ-FeO (OH). There are studies in which the authors compare the rust taken for analysis in various places (coastal, continental, etc.). The rust formed in the coastal areas was mainly in the form of large flakes; in areas with high moisture and chlorides in the soils, leaf-shaped rust was formed, and powdery and fine-grained rust was the lot of the central and northern territories. Layered rust samples contained γ-Fe 2 O 3 · H 2 O on the contact surface with air and Fe 3 O4 on the metal contact surface, α-FeOOH and δ-FeOOH were found in the intermediate layers and in flakes.
Why am I telling all this, and then that I need to know the enemy in person. The more accurately determine the type of rust - the more efficient it can be dissolved.
It is well known that depending on environmental conditions, multi-colored rust may form: red rust (hydrated oxide Fe 2 O 3 · H 2 O is formed at high levels of oxygen and water vapor, most often it is uniform atmospheric corrosion in very aggressive environments.), yellow rust (so-called solvated rust, soluble FeO (OH) · H 2O образуется в условиях высокой влажности, чаще всего, если металл находился в луже/стоячей воде), бурая ржавчина (сухой оксид Fe2O3, который образуется при высоком содержании кислорода и низкой влажности, чаще представляет собой локализованную ржавчину, которая проявляется в виде неоднородных пятен или только в определенных областях (загрязнения и дефекты на поверхности металла) и черная ржавчина (оксид Fe3O4 который образуется в среде с низким содержанием кислорода и низкой влажности, является устойчивым видом ржавчины, похожим на слой покрытия, возникающего при оксидировании металла).

If ions of various inorganic acids come into play (carbonates, sulfates, chlorides already mentioned, as well as bromides, fluorides, iodides, nitrates and selenates), so-called green rust

Green rust is the common name for various crystalline green compounds containing iron cations and the anions mentioned above. This beauty (?) Is formed on iron / cast iron / steel surfaces exposed to water containing chloride, sulfate, carbonate or bicarbonate ions during alternating aerobic / anaerobic conditions. For example, in ships, bridges, etc. A general view of the crystal structure of green rust is shown in the picture. In principle, we can assume that the structure of the usual “shades of red” rust is in many ways similar to green, but without acidic anions.

Although rusting is generally a negative aspect, there are examples where it also serves the person. An example is the bluing of weapon trunks (including processing with the so-called “rust varnish”) and homemade knife blades.
Gun barrels after rust treatment
This is how they look right after processing:

And so - after polishing:


And so - after polishing:

A thin oxide film of “black rust” (described above) is formed on the metal, protecting the metal from further oxidation. This process is also called oxidation:
Oxidation - the creation of an oxide film on the surface of a product or workpiece as a result of a redox reaction. Oxidation is mainly used to obtain protective and decorative coatings, as well as for the formation of dielectric layers.
As an example, we can mention the iron column (aka Kutubova column ) in Delhi - a cylinder seven meters high and weighing six and a half tons, which is part of the Qutb Minar architectural ensemble, located about 20 kilometers south of Old Delhi. The column gained fame by the fact that for 1600 years of its existence it virtually avoided corrosion.

What they didn’t expect about this column, from “made of meteorite iron” to “gift of Shiva”. But, traditionally, the answer was simple - “high temperature and dry air”
Threaded Rust as is
If we briefly introduce the mechanism of thread rusting, it turns out that it practically does not differ from metal rusting on the surface. Initially, water enters the inter-threaded channels (between the nut and the bolt), which, in combination with air oxygen and iron electrons, starts the processes described by the formulas above. As a result of this process, iron oxides and hydroxides begin to form, which, depending on the conditions, undergo hydration / dehydration cycles and form a monolithic porous structure. It can be said that rusting inside a thread differs from rusting an open metal in that a lack of oxygen can be observed inside the thread and something similar to black rust will form (Fe 3 O 4 ).
Probably the closest phenomenon to “souring” is rusting of reinforced concrete. The same conditions lack oxygen. Under such conditions, the volume of loose oxides formed as a result of oxidation is much larger than the volume of the reacted metal. Oxides completely fill all pores and leaks (threads or protrusions near the reinforcement), acting as a kind of proppant or sealant.

As a result of the described process, slowly but inexorably true, rust presses everything in contact with it and destroys concrete, blocks the thread, etc. There is even such a thing as “rust packing” or “pack rust”, which in translation into the great and mighty means “packet rust”. It is observed in those cases when the volume of the formed oxides with its wedging pressure squeezes out metal parts adjacent to the places of corrosion. The most famous example of the effects of batch rust can be the collapse of the bridge over the River Myanus in the United States in 1983 with many casualties.

A special investigation found that corrosion resulting from rainwater entering the metal structures of the bridge due to impaired drainage technology gradually leaked to the iron mounts. The fasteners gradually rusted and pushed one corner of the road slab from the support in millimeters. When the distance became sufficient for destruction, one passing car served as a trigger. Since then, a new term has appeared in bridge construction and is actively used when signs of rust accumulation between steel plates and bridge joints are observed during inspection of the bridge
Concrete crushed, but can not handle the bolt

I hope the mechanics of the process are approximately clear. It is needed in order to evaluate the existing methods of dealing with threaded (almost "batch") rust.
Methods for the destruction of rust inside a thread
In the simplest way, in order to unscrew the bolt you need to do two things
- Destroy (= disperse) the monolithic porous mass of oxides and hydroxides with the formation of areas of reduced density, “defects” and cavities
- Reduce friction between fragments of monolithic oxides and allow them to easily slide relative to each other along with turning the nut
This can be done by various methods:
destruction_method 1 - Mechanical
In fact, grandfather. Because for a long time, in the absence of other possibilities, it is customary to knock on a rusted thread in the hope of destroying monolithic plates and chains of formed oxides with vibration. As soon as the bolt is removed, the oxides (and they are fragile enough) will continue to rub themselves into dust. The method is not particularly effective and, in addition, requires a specialist who "feels a hammer", so as not to inadvertently twist or rivet a bolt tightly.
A good option is to use electric or pneumatic impact wrenches (+ there are also screwdrivers, added redbeardster in the comments ), like the one in the picture:

Although in this case, as well as with an ordinary hammer, the main thing is not to overdo it and not break the bolt. It is better to install a correctly selected head from the side of the nut, and at the same time support the bolt with an additional wrench.
In case there is no need to save the fasteners (for example, in the case of antique cars), you can simply cut the nut with an angle grinder (grinder), and drill the bolt. But the labor costs for such a procedure are such that I would advise using this method in the last turn, having tested all the possibilities described in the article.
Addition: I remembered about the grinder , but about the wrenches (they are nut cutters and nut cutters) - no. Thanks to the p_fox reader who reminded me of such a thing.

destruction_method 2 - Thermal
This method is based on the understanding that all bodies expand when heated, and when cooled, they contract. Heating the nut (or the object into which the bolt is screwed) will lead to the formation of micro-fractures in the oxide monolith located along the thread. The alternation of heating / cooling cycles is very likely to lead to crushing of rust flakes and the formation of "vacuum". And as soon as holes are formed inside the layer, the bolt will most likely turn around. In addition to mechanical crushing of the oxide layers due to the expansion of the metal, dehydration of the rust components themselves takes place. For example, annealing at 350 ° C turns rust mainly into maghemite (γ-Fe 2 O 3), magnetite forms at 550 ° C, and at 750 ° C the rust turns into a mixture of magnetite, wustite (FeO) and metallic iron (Fe).

Previously, this method was available only to owners of acetylene or propane burners, but with the advent of aliexpress today, almost everyone can buy a compact burner for a “dichlorvos” cylinder and calcine rusted bolts and nuts for their own pleasure.

Actively thermal removal of rust is also used in the restoration of iron products. True, this is not just annealing, but the high-temperature reduction of oxides into elemental iron. This is done either by heating the rusted products in a carbon monoxide medium (aka CO, aka carbon monoxide) under a layer of charcoal with limited air access and a temperature of 800 ° C. Hydrogen can also be used as a reducing agent, especially if there is access to tube furnaces with temperature controlled along the length of the furnace. Ammonia is fed into the reaction part of the furnace, which decomposes on the catalyst at 400-600 ° C into nitrogen and hydrogen. Hydrogen reduces oxides to “sponge iron”, which further requires additional treatment with protective agents, such as molten paraffin.
Addition:In the same section I will introduce the laser rust cleaning feature mentioned by the Alexus819 reader , which is perfectly applicable for smooth surfaces (see clickable video).

destruction_method 3 - Chemical The
chemical method for the destruction of threaded rust is based on the fact that chemical components entering the pores and capillaries of the oxide layer can interact with it, either converting rust into a soluble compound or reducing it to metallic iron. Both relieve the proppant pressure inside the thread and allow the nut to be rotated due to the formation of additional pores or areas of reduced density. In general, the mechanisms of influence of the chemical method can be divided into three directions: protonation , complexation , reduction. No wonder I quoted the names of the most common “minerals” forming rust at the beginning of this article. I did this so that an inquisitive reader could pick up a suitable reagent, hidden in a table under the spoiler.
And now, a little detail about each of the mechanisms with examples.
Protonation
As a result of protonation, reagents capable of becoming a proton donor (H + ) react with rust . Most often, mineral inorganic acids are used for this purpose.
The mechanism of protonation of Fe (III) under the influence of acids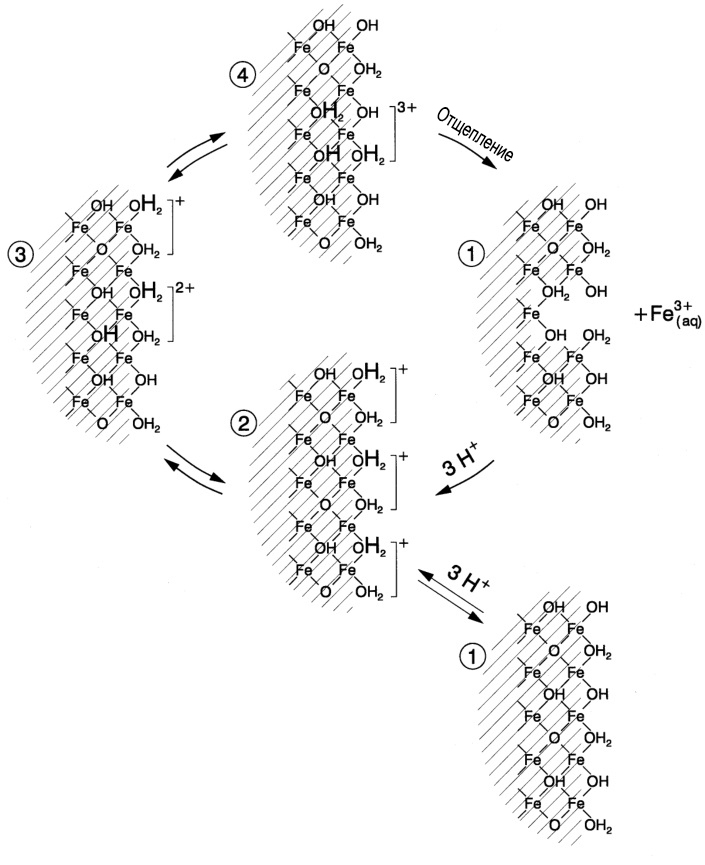

Traditionally, solutions of mineral acids are used to clean the surface of iron from corrosion products. The most active is a solution containing 35% orthophosphoric and 5-10% hydrochloric acid. Solutions of acids - sulfuric, hydrochloric - allow you to quickly remove corrosion products, but always cause some metal pickling. To prevent this, corrosion inhibitors are introduced into acid solutions. So, in 1 M (I recall, just in case, that a solution of a concentration of 1 M contains 1 mol of a substance per liter of solution) sulfuric acid solution, it is advisable to add thiosemicarbazide, thiourea, urotropin, triphenylphosphine, benzotriazole (good results are achieved when iron is treated with a 1 M sulfuric acid solution containing 0.1-0.5% thiourea or 0.5-1.0% benzotriazole); in 1 M hydrochloric acid solution - urotropine and triphenylphosphine.
Note: Triphenylphosphine is an organic compound with the formula P (C 6 H 5 ) 3 , or simply Ph 3 P. It is a derivative of phosphine. It has the appearance of white crystals. Relatively stable when stored in air.
Here is such a beauty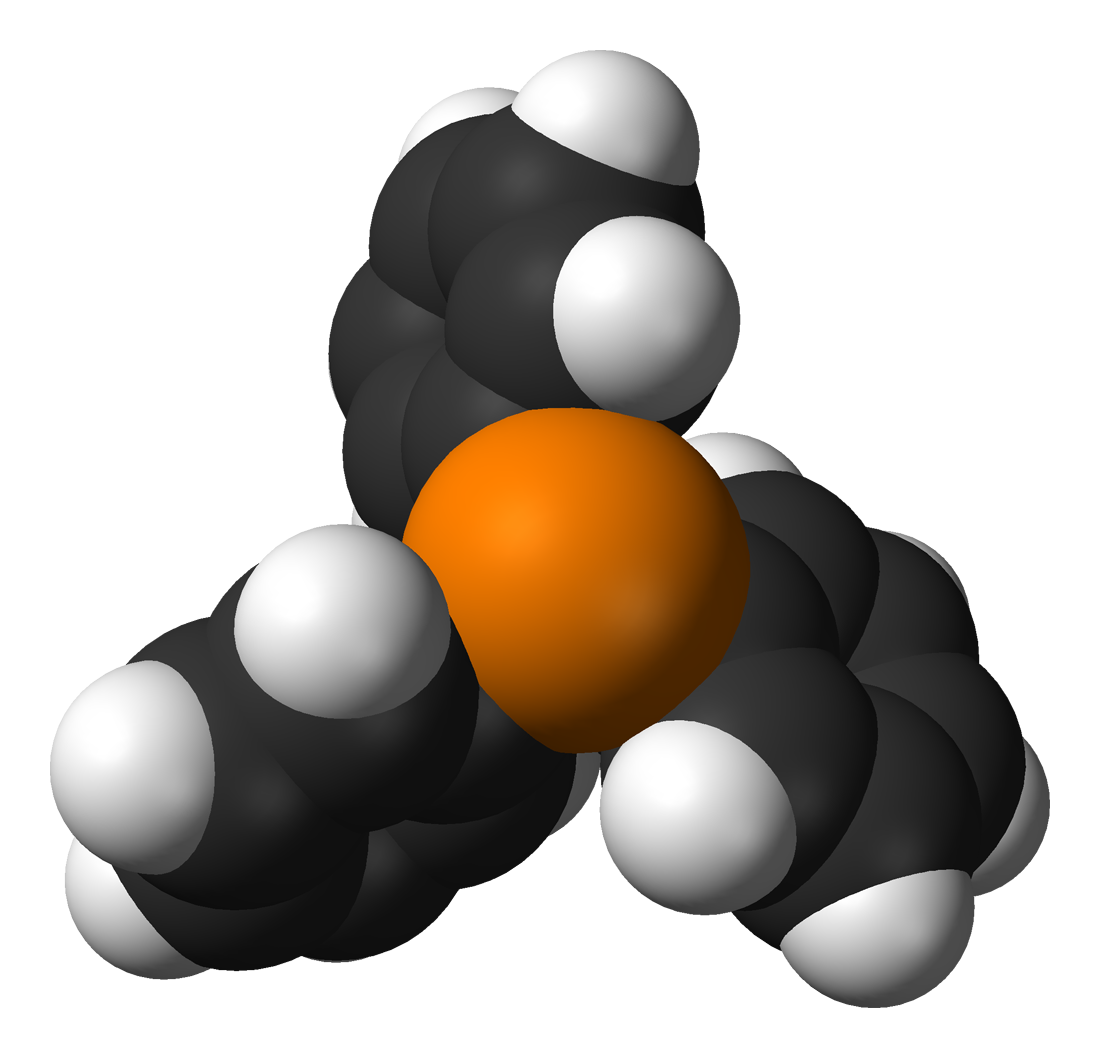

Triphenylphosphine is also interesting because its derivative, triphenylphosphine oxide, is widely used in microelectronics, and by its smell, Labrador dogs in the USA are taught to find various electronic memory devices. Under the spoiler excerpt from Science and Life No. 10/2018

It smells like electronics
The use of sniffer dogs to search for criminals, explosives, drugs has a long history, but these days it turned out that the dog can be dragged to electronics, right down to flash drives. In the United States, only 17 dogs have been trained so far (data for February 2018), all of them Labradors. Their search objects are not only flash drives, but also cell phones, hard drives, SD memory cards, microSD, SIM cards for phones, tablets and other equipment with illegal content that they are trying to hide from the authorities. The total area of olfactory receptors in humans, if placed in one layer, is approximately a postage stamp, in a dog - a table napkin. But only one out of every 50 dogs can be taught this art.
It takes half a year to train bloodhounds to search for electronics. Forensic scientists have done research - what can computer memory devices smell like? It turned out that all of them contain triphenylphosphine oxide, a substance that is used in the production of nanoelectronics. For humans, this white powder has no smell, but four-legged detectives feel it. Dogs are taught to associate this smell with feeding, and right up to “retirement” they are fed only by search results. However, once a week the dogs are given a rest and fed just like that, without searches.
The training program and the use of “detectives of electronics” was kept secret for two years, fearing that criminals would find some way to mask the smell or counteract the dog’s scent, but recently this information has been declassified. It turned out that it doesn’t even help to hide the microcircuit in a spray can with shaving cream, which has its own distinct smell.
It takes half a year to train bloodhounds to search for electronics. Forensic scientists have done research - what can computer memory devices smell like? It turned out that all of them contain triphenylphosphine oxide, a substance that is used in the production of nanoelectronics. For humans, this white powder has no smell, but four-legged detectives feel it. Dogs are taught to associate this smell with feeding, and right up to “retirement” they are fed only by search results. However, once a week the dogs are given a rest and fed just like that, without searches.
The training program and the use of “detectives of electronics” was kept secret for two years, fearing that criminals would find some way to mask the smell or counteract the dog’s scent, but recently this information has been declassified. It turned out that it doesn’t even help to hide the microcircuit in a spray can with shaving cream, which has its own distinct smell.
Since we started talking about inhibitors, in addition to the ones mentioned above (and available in the ASTM tables below), we can also mention various amines that are found in branded “rust solvents”. Monoethylamine, diethylamine, triethylamine, etc. are actively used. (which is at hand).
Complexation
Complexation - the process of the so-called coordination compounds. They are neutral molecules or ions resulting from the addition of neutral molecules or other ions called ligands to an ion or atom called a complexing agent. Most often, ligands act as bulk organic molecules.
The general mechanism of dissolution of rust through complexation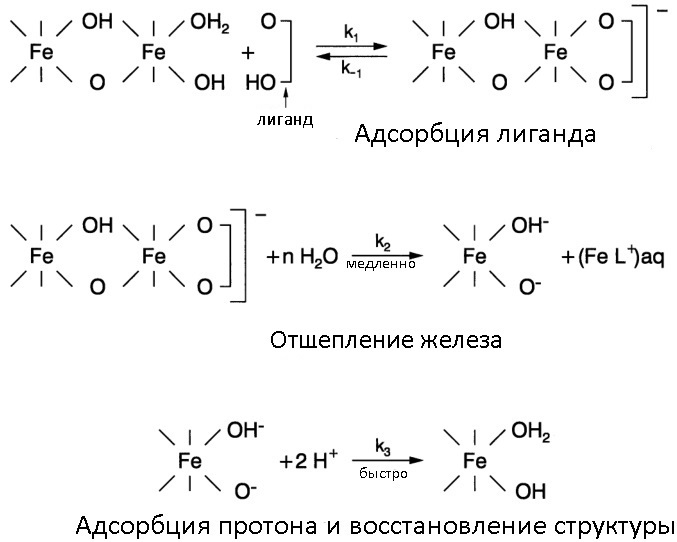

Most of the organic acids used in dissolving rust work precisely according to the mechanism of complex formation with Fe (III) ions. The best efficacy is shown by formic, citric and oxalic acid (as well as their salts), ethylenediaminetetraacetic acid (EDTA) and its salt Trilon-B, which is actively used in all kinds of Calgons to remove scale. When using solutions of organic acids, corrosion inhibitors (the same urotropin) can be added to their composition, since acids, albeit slowly, but still sometimes cause metal pickling. I would like to note that organic acids work better in the presence of small amounts of mineral acids (pH adjuster) and when heated (see the picture under the spoiler).
Comparison of the dissolution efficiency of iron oxides with citric and oxalic acids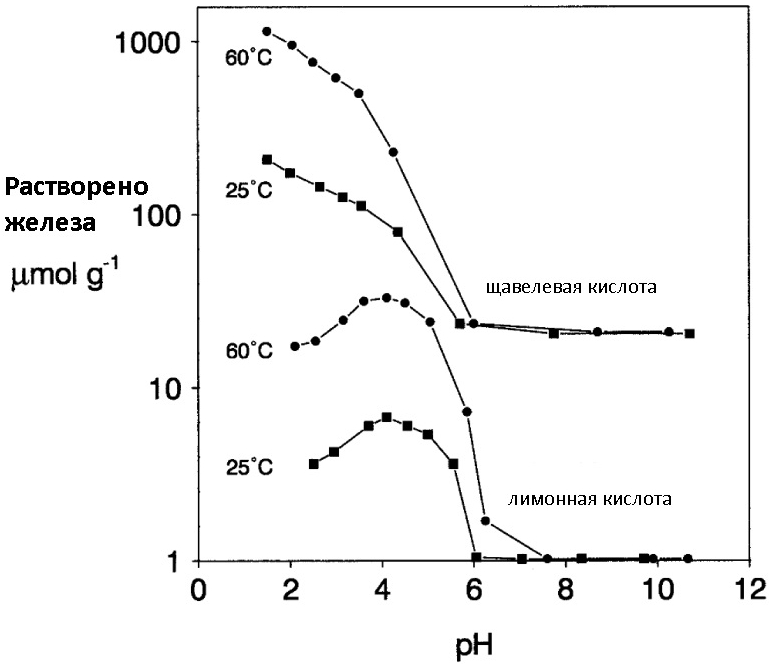

Recovery
Well, and finally, the third mechanism by which structural bonds between iron atoms in iron oxides can be weakened and destroyed is the reduction of structural Fe (III) to Fe (II) (or even Fe 0, if you're lucky). Its essence lies in the processes of electron transfer and the related adsorption of an electron donor, cathodic polarization of the electrode, electron transfer from unstable surface complexes to the Fe (III) surface, and much more matan. More important, in my opinion, is that a huge number of compounds are used as reducing agents - sodium dithionite (bisulfite), thioglycolic acid (it is also mercaptoacetic, widely used for perming and dyeing hair) acid, thiocyanate, hydrazine, ascorbic acid, hydroquinone, hydrogen sulfide fructose, sucrose. And even such amazing things as sulfonic acids from the soil:

Under appropriate conditions, reductive dissolution can also be carried out photochemically.
The most popular reducing agents are hydrogen peroxide, a 3-5% aqueous solution of NaOH and sodium sulfite Na 2 SO 3 , the sodium bisulfite already mentioned above, ascorbic acid. Often, zinc powder in a 15% NaOH solution (which, by the way, when applied to smooth rusty surfaces, is additionally thickened with polyvinyl alcohol) is used as a reducing solvent for rust
. Comparison of the effectiveness of various methods for dissolving rust
Often, to dissolve rust, not individual reagents are used, but their combinations, where each substance implements its own mechanism for dissolving iron oxides. An example is the same zinc + NaOH in which Trilon B is added to speed up the process, thereby regulating the cleaning ability of the mixture. Below the spoiler is a picture with which you can compare the dissolution rate of rust using various mechanisms.
Comparison of the efficiency of dissolution of oxides using different mechanisms (protonation, complexation, reduction)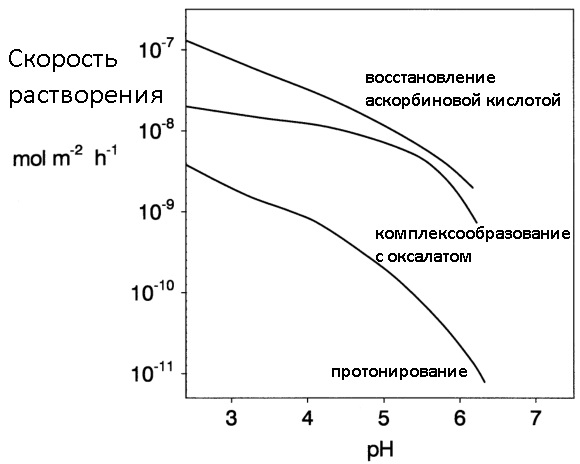

Below is a diagram that shows that rust dissolution methods using combinations of different (heterogeneous) “oxide layer destroyers” are most effective.
The efficiency of the dissolution of iron oxides using combined approaches compared to traditional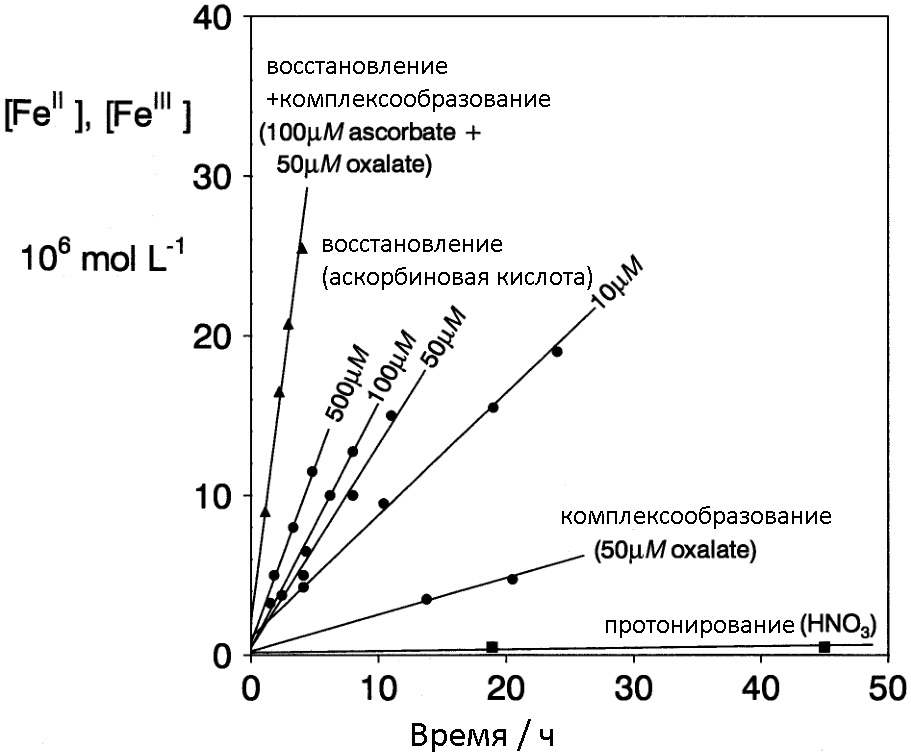

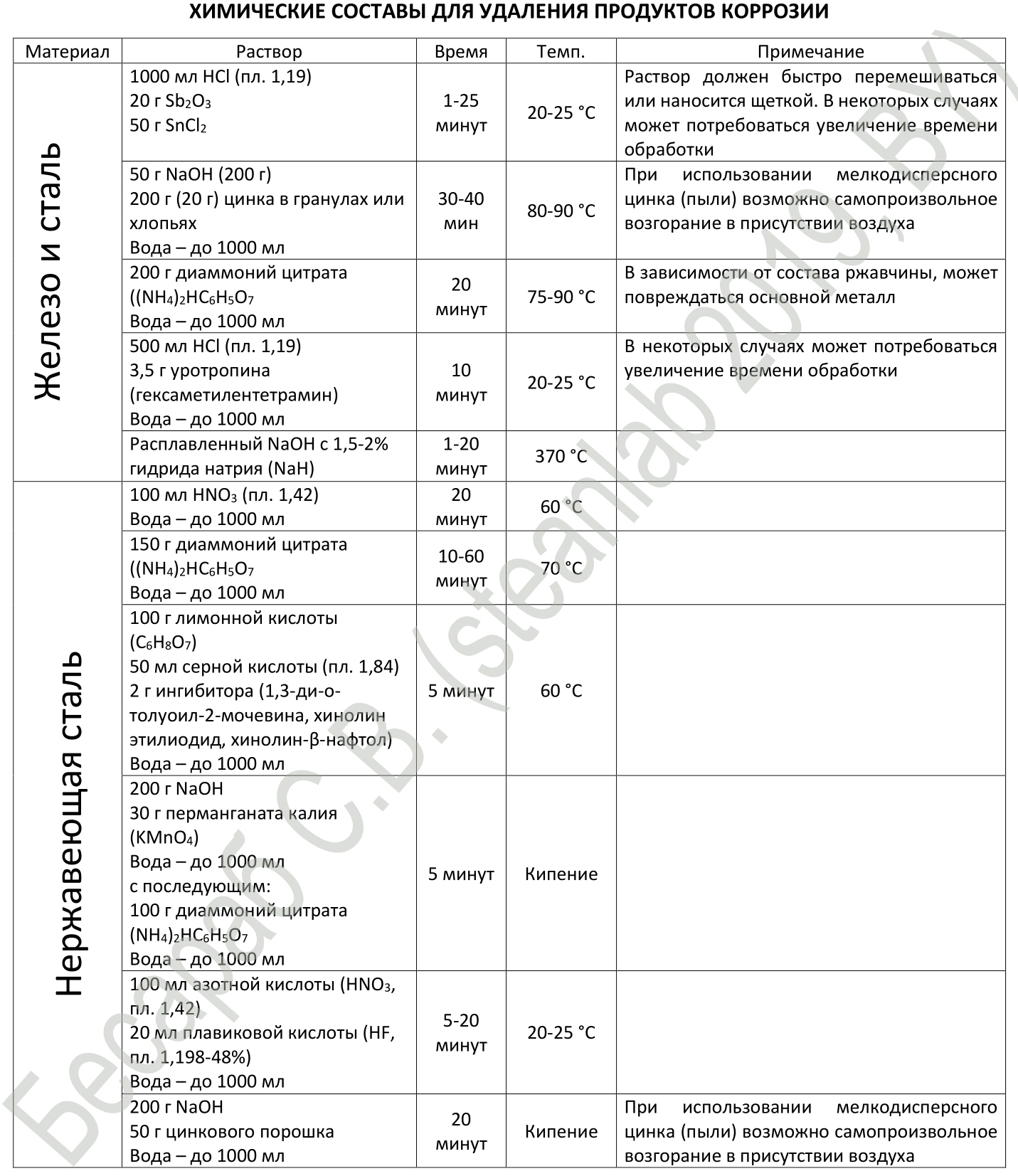
Well, to summarize, I will give a methodological recommendation, taken from the American ASTM standard on the removal of corrosion products from metal. The Americans themselves, as I understand it, most likely focused on the processing of materials on the plane, but everything below can easily be applied to threaded joints.
ASTM Recommended Chemical Corrosion Removal Compounds
For chemical cleaning processes:
And, for lovers of aquick ride of electrolysis - compounds for electrochemical cleaning. The process is really seriously accelerated compared to chemical treatment.


And, for lovers of a

Note from a colloidal chemist : it must be understood that in the case of a thread (as opposed to rust on the surface), in order to destroy all the oxides along the thread, the above compounds must reach them (the reaction proceeds only at the place of direct contact between the reactant and the oxide). And to do this is quite difficult, since all penetration paths are tightly clogged with rust that has not yet had time to react. Therefore, in addition to the dissolution efficiency, the wetting effect of the reagent (the ability to penetrate into pores, microcracks, and capillaries) should be taken into account. The following section is devoted to these issues.
Friction reduction or “lubricating debris ...”
As I wrote above, after chemical, thermal or mechanical destruction of the oxide layers of rust, it is necessary to reduce the friction between them. It is logical that this can be done using grease. Most “folk” remedies, such as various oils, kerosene, gasoline, acetone, do not change the state of rust inside the “sour” bolt, but they can help themselves in turning the nut together with the fragments of the oxide layer after the initial treatment to destroy the porous frame.
Important!Pouring any hydrocarbons onto the “acidified” thread makes sense only if the path along the thread is not completely clogged with oxides, in which case the solvent penetrates / adsorbes into them and only then acts as a lubricant. Those. it is necessary to moisten with all kinds of kerosene either the bolts that are not very rusted or when the bolt has already been touched and pores have formed in the oxides. Therefore, given the foregoing (for example, the paragraph thermal destruction) it would be logical to apply “kerosene” to the rusted thread, and then simultaneously with tapping the bolt head with a hammer or other percussion instrument, try to move the nut from its place. For cast-iron and steel water pipes with a thread, experts recommend heating the rusted places, applying paraffin from a candle, heating them again until the melted hydrocarbon is absorbed and slipping along the threads and only then try to unwind.
A note about the WD-40.Many probably heard about this thing, the "triumph of American petrochemicals." I, unlike some friends who cannot imagine a car without a WD-40 bubble in the glove compartment, do not feel much reverence for this mixture of hydrocarbons. For those who are not in the know, this is a solution that was developed in the 60s of the last century to protect the corps of American missiles from rust and corrosion. Well, then, as usual, ordinary Americans appreciated the benefits of this incomprehensible liquid. "WD-40" is an abbreviation for the term "water displacement, 40th formula", i.e. for the 40th time they did something. Composition WD-40 has never been patented, in order to preserve trade secrets. Therefore, it is still not really clear what is in the original development. Every year it’s becoming more and more difficult to find out (because according to the stories of knowledgeable people>brought this "kerosene" onto a gas chromatograph + mass spectrometer and found that it contains: mineral oil, decane, nonane, undecane, tridecane, tetradecane, cyclohexane, dimethylnaphthalene and carbon dioxide to create the necessary pressure in the cylinder. MSDS (safety data sheet for the US market) gives the following information: 50% - aliphatic hydrocarbons, <25% - mineral oil, 12-18% aliphatic hydrocarbons with low vapor pressure to reduce the viscosity of the solution (easily volatile diluent), 2-3% carbon dioxide, <10% inert ingredients.
The solution mentioned above is often praised for its incredible permeability (or permeability, I don’t remember exactly how WD fans say it). If you look from the point of view of colloid chemistry, it turns out that these people most likely mean the phenomenonwetting . Briefly, it depends on the forces of intermolecular interaction and consists in the following: if the forces of interaction between molecules of a liquid and a solid are greater than between molecules of a liquid, then the liquid spreads over the surface of the solid, i.e. it wets and vice versa, if the interaction forces between the liquid molecules are greater than between the liquid and solid molecules, then the liquid collects in a drop and does not wet the surface of the liquid. This is directly related to such a thing as surface tension .
Tatko napaminae : so that the son does not forget to mention such a thing as “red tormozuha”, it’s also brake fluid BSK (like “butyl-alcohol-castor oil” in a 1: 1 ratio).
Soviet brakes, inhibits corrosion better than anyone in the world

While someone was using the WD-40 from the glove compartment, if I was disassembling a motor and suddenly there was a tightly rusted fastener, the following happened. Tatko silently looked at this matter, then silently went to the garage and brought a syringe with a red liquid of pungent odor. The liquid was applied, aged 30 minutes and ... And indeed, in most cases it worked and the nut could be unscrewed. In fairness, I note that all the fasteners for which BSK was used were in motors, where there was always a certain amount of lubricant. To be honest, it is very likely that my father still has this brake fluid in the garage, which is stored specifically in case of rusted fasteners. It’s hard to find such an option on sale now, because manufacturers abandoned butanol in favor of various polyglycols and their esters, which penetrate the thread capillaries much worse. Perhaps this is due to the fact that polyglycols are cheaper, and maybe because they are safer.
Addition: @ Alexey Shukaev clarifies that the transition from butanol to polyglycols is associated with a difference in boiling point. "The transition to gaseous => compressibility => the hydraulics stops working" - so I had to refuse ...
In my memory there were examples of cases when people, tired of finding money for factory booze, drank red brake fluid. Butanol, after all from the same series of alcohols as ethanol, even though it has the highest toxicity among simple alcohols (LD 50is 2290-4360 mg / kg). Most fusel oils in alcohol production by distillation are butanol. It is he who gives the furious, incomparable hangover. But this is when "digested." And castor oil, it is known for its therapeutic laxative effect. In general, a multifunctional product was produced in the USSR ...
Note : in the modern world, tired of the unsuccessful search for a mixture of castor oil and butanol, its analogue (of the same color) is used: transmission (red) oil from an automatic transmission (ATF) and acetone in a ratio of 1 :1. Color similar, efficiency too.
When contacting alcohol, remember
… что легендарный советский физик Лев Давидович Ландау об алкоголе говорил следующее: «Традиционно выпитый новогодний бокал шампанского на целый месяц лишает меня творческой активности». А друзья его, например выдающийся швейцарский физик Вольфганг
Паули (нобелевский лауреат, кстати) дополняли: «Я знаю, почему Ландау не пьет. Он пьян всегда. Он опьянен самой жизнью, ему не нужен алкоголь». Вот на кого, ребята, нужно равняться.
Паули (нобелевский лауреат, кстати) дополняли: «Я знаю, почему Ландау не пьет. Он пьян всегда. Он опьянен самой жизнью, ему не нужен алкоголь». Вот на кого, ребята, нужно равняться.
И что же из всего этого следует ?
And from all this it follows that if a liquid (some solvent) has a surface tension less than water, then it will be better to moisten the rust and penetrate into the pores and capillaries faster than an aqueous solution of any acid. It will be better to penetrate, but it will not be able to destroy or weaken the bonds between the oxide layers. The following observations are emerging:
1) WD-40, and all kinds of “liquid keys” (English liquid wrench) - are ordinary hydrocarbons and components close to them, which have low surface tension and are able to wet porous oxides well and penetrate their capillaries.

These, as a rule, oil products, are perfectly absorbed by thread threads and provide lubrication. Only lubrication, because all components themselves are inert and do not have any noticeable effect on rust. From the word in general. Therefore, it is best to use them after / in conjunction with the methods of destruction of oxide layers described in the article. The sad thing is that even RUST REMOVERs recognized by rust would not corrode and penetrate perfectly.
2) Any PB-Blaster, Rust buster and Rust dissolver declared as a solvent for rust, really dissolve rust. As a rule, they also contain a component that reduces surface tension and gives a light lubricating effect. But this effect is deeply secondary. Under the spoiler a couple of famous examples:
Rust solvents inside
Первым у нас идет продукт от Henkel — Loctite Naval Jelly Rust Dissolver

Cостав:
Ортофосфорная кислота — 10.0-30.0 % (= протонирующий растворитель ржавчины + фосфатирующий металл агент)
Изопропанол — 1.0-5.0 % (= «смазка» т.е. компонент обеспечивающий смачивание и проникновение)
Серная кислота — 0.1-1 % (= протонирующий растворитель ржавчины)
Вода — >50 %
Полисахариды — ? (= загуститель для создания правильной консистенции)
Далее некий Permatex Rust Dissolver Gel

Состав:
Вода — 40.0-70.0%
Фосфорная кислота — 10.0-30.0 % (= протонирующий растворитель ржавчины + фосфатирующий металл агент)
Изопропанол — 1.0-5.0 % (= «смазка» т.е. компонент обеспечивающий смачивание и проникновение)
Моноэтаноламин (MEA) — ? (= ингибитор коррозии)
Родамин — ? (= краситель)

Cостав:
Ортофосфорная кислота — 10.0-30.0 % (= протонирующий растворитель ржавчины + фосфатирующий металл агент)
Изопропанол — 1.0-5.0 % (= «смазка» т.е. компонент обеспечивающий смачивание и проникновение)
Серная кислота — 0.1-1 % (= протонирующий растворитель ржавчины)
Вода — >50 %
Полисахариды — ? (= загуститель для создания правильной консистенции)
Далее некий Permatex Rust Dissolver Gel

Состав:
Вода — 40.0-70.0%
Фосфорная кислота — 10.0-30.0 % (= протонирующий растворитель ржавчины + фосфатирующий металл агент)
Изопропанол — 1.0-5.0 % (= «смазка» т.е. компонент обеспечивающий смачивание и проникновение)
Моноэтаноламин (MEA) — ? (= ингибитор коррозии)
Родамин — ? (= краситель)
As you can see, all the same vigorous inorganic acids and alcohols are used to give them the necessary mobility and reduce surface tension. + In some cases, corrosion inhibitors. Those. theoretically, everyone can experimentally make their own rust solvent by mixing their favorite (= available) inorganic acid with alcohol (available).
Important : all the chemical methods of rust destruction and transformation described in the article can be used not only to dissolve “sour” threads on bolts, but also for anticorrosion treatment of metal (iron / steel / cast iron) of any shape, as well as when removing rusty stains on plumbing etc.
Well, for a snack I would like to offer such a fact. Recently, in connection with the desire of manufacturers of chemical compounds to comply with the concepts of green chemistry , constantly searching for new, more environmentally friendly and biodegradable components. All kinds of solvents and rust converters did not stand aside. The latest trend is the use of organic compounds of a phenolic nature, tannins, as a transforming component (instead of the usual phosphoric acid and iron phosphate, for example). The tannin action of these substances turns the reddish iron oxides into a bluish-black stable tannate. Here you have a place where you can attach astringent persimmonwith her tannins :). And in general, theoretically, it’s quite an option to treat the rusty bottom of your favorite car, instead of toxic inorganic acids, with a strong infusion of green tea ...
This is the end of the rust story, subscribe to my Facebook / VKontakte notes to know more and be in the subject of the latest research!

References
Никитин М.К. Химия в реставрации. – Л.: Химия, 1990. – 304 с.
ASTM G1 — 03(2017)e1 Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens
www.armorvci.com/corrosion/types-of-rust
T. Kisato, Rust conversion agent for corrosion prevention. Japanese patent 2014, JP 2014169486 A 20140918.
M. Usman, J.M. Byrne, A. Chaudhary, S. Orsetti, K. Hanna, C. Ruby, A. Kappler, S.B. Haderlein, Magnetite and green rust: synthesis, properties, and environmental applications of mixed-valent iron minerals, Chem. Rev. 118 (2018) 3251–3304.
R.M. Cornell, U. Schwertmann, The Iron Oxides: Structure, Properties, Reactions, Occurrence and Uses, 2nd ed., Wiley-VCH, 2006.
Y.S. Choi, J.G. Kim, Aqueous corrosion behavior of weathering steel and carbon steel in acid-chloride environments, Corrosion 56 (2000) 1202–1210.
Hansen, H. C. B. Environmental Chemistry of Iron(II)-Iron(III) LDHs (Green Rusts). In Layered Double Hydroxides: Present and Future; Nova Science Publishers: Huntington, NY, 2001; pp 469−493.
Réguer,S.,Dillmann,P.,Mirambet,F.:In:Dillmann,P.,Béranger,G.,Piccardo,P.,Matthiessen,H.(eds.) Corrosion of metallic heritage artefacts, p. 170. Woodland Publishing Ltd., Cambridge (2007)
web.archive.org/web/20140119014037/http://www.wired.com/science/discoveries/magazine/17-05/st_whatsinside
Lange's Handbook of Chemistry (1967) 10th ed. pp 1661–1665
ASTM G1 — 03(2017)e1 Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens
www.armorvci.com/corrosion/types-of-rust
T. Kisato, Rust conversion agent for corrosion prevention. Japanese patent 2014, JP 2014169486 A 20140918.
M. Usman, J.M. Byrne, A. Chaudhary, S. Orsetti, K. Hanna, C. Ruby, A. Kappler, S.B. Haderlein, Magnetite and green rust: synthesis, properties, and environmental applications of mixed-valent iron minerals, Chem. Rev. 118 (2018) 3251–3304.
R.M. Cornell, U. Schwertmann, The Iron Oxides: Structure, Properties, Reactions, Occurrence and Uses, 2nd ed., Wiley-VCH, 2006.
Y.S. Choi, J.G. Kim, Aqueous corrosion behavior of weathering steel and carbon steel in acid-chloride environments, Corrosion 56 (2000) 1202–1210.
Hansen, H. C. B. Environmental Chemistry of Iron(II)-Iron(III) LDHs (Green Rusts). In Layered Double Hydroxides: Present and Future; Nova Science Publishers: Huntington, NY, 2001; pp 469−493.
Réguer,S.,Dillmann,P.,Mirambet,F.:In:Dillmann,P.,Béranger,G.,Piccardo,P.,Matthiessen,H.(eds.) Corrosion of metallic heritage artefacts, p. 170. Woodland Publishing Ltd., Cambridge (2007)
web.archive.org/web/20140119014037/http://www.wired.com/science/discoveries/magazine/17-05/st_whatsinside
Lange's Handbook of Chemistry (1967) 10th ed. pp 1661–1665