Powder for the brain or how to make powder for the dishwasher 9.7 times cheaper
- Tutorial

Update
A new version of the powder and a more detailed analysis in the second part: DIY powder for the dishwasher: we disassemble industrial products and improve the recipe
Now I’ll tell you how to make powder for the dishwasher from soda and washing powder. The same in composition, only an order of magnitude cheaper.
There are so many areas of our lives where our presentation forms exclusively marketing bullshit. Alas, most people do not even try to think about what underlies all this. Very often, the market situation leads to the fact that the cost of the product is 0.5% of its price. The rest is marketing, margins, logistics, packaging and the like. Almost everyone is aware of the concept of selling ink for a printer at the price of tears of Himalayan virgins and the persistent recommendations of manufacturers to use only original supplies. For example, it recently dawned on me that 1.5 grams of dry matter in a bottle of fertilizer for plants cannot cost 200-250 rubles. But it is precisely such an amount that can fit in a relatively stable state in the form of a solution. I immediately imagined hectares of fields and trucks,tons of powder . As a result, I switched to packaging of 1 kg of Buisk complex dry fertilizers. You can prepare a bath of solution.
Today we will create ultra-cheap powder for dishwashers. The reduction in real wages and the rise in price of imported household chemicals forced me to search through textbooks, look at a bunch of materials from chemist forums and try to find ways to save on consumables. Powder became very noticeable. The results of thoughtful research and experiments were very surprising. For industrial applications, chemists and technologists most often create individual recipes depending on the quality of the water and the tasks. Why don't we try to figure it all out?
TLDR:
70% calcined soda and 30% washing powder instead of detergent.
If you are too lazy to mess with soda, then just Biolan powder or its analogues. Soda makes it cheaper.
Edible salt instead of salt.
Types of pollution
What do we need from the powder? Weird question. To wash. Well, it is desirable that it is not very poisonous and does not dissolve dishes with the dishwasher. Detergent should cope with the main types of pollution:
- Fat. The main pollutant is all kinds of cooking oils, salad dressings, fingerprints, as well as fatty sauces and more.
- Protein pollution. They are less common, but difficult to remove - eggs, cereals and other similar options.
- Dyes. Tea, coffee, beets and other highly coloring things.
- Smell. A chicken that has been forgotten in the container for a couple of weeks can give you a lot of thrills.
- Inert mechanical pollutants. Sawdust, adhering pieces of greenery, radioactive dust and every little thing.
- Unknown burnt garbage from the party before last. Nothing will help. Do not expect. Throw away the dishes.
Various surfactants (surfactants) do a good job with the first type of pollution. These are chemical compounds that, when concentrated on the interface of thermodynamic phases, cause a decrease in surface tension. Simplistically, one can imagine this as enveloping particles of fat with surfactant molecules and preventing them from sticking back to the surface.
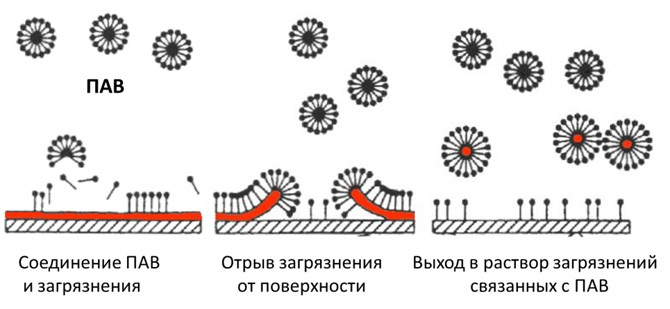
This class of substances is quite extensive and includes the main two groups: ionic (anionic) and nonionic surfactants. The former decompose in solution into ions (salts of fatty acids, for example, ordinary liquid soap is potassium salts), the latter do not dissociate (alkyl glycosides and others). Anionic, as a rule, wash better, but are more aggressive.
Protein contamination is a bit more complicated. Traditionally, dishwasher powder includes enzymes (enzymes) that hydrolyze protein into small peptide pieces. Enzymes are capricious, they work in a small range of pH and temperature, since they themselves are a protein. At a high washing temperature (more than 60 degrees), almost all of them will be denatured and stop working. An alternative that is more characteristic of industrial facilities is the creation of an alkaline environment. Many used a pipe cleaner like the Mole and the like. As part of detergents (surfactants) and alkali (sodium hydroxide). All this infernal mixture destroys almost any organic matter, including proteins and fats. Proteins break down into individual peptides and amino acids. Fats turn into soap:
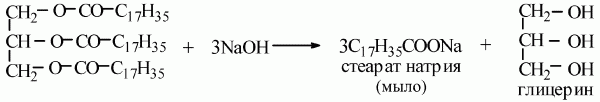
The same reaction underlies one of the ancient methods of obtaining soap from vegetable and animal fats. The main disadvantage is that a very aggressive environment is obtained. Concentrated alkali will also joyfully dissolve the skin on the hands. Caution is required in use.
With dyes, everything is quite simple. As a rule, to discolour a pigment, it is sufficient to oxidize it by changing its structure. Most often this is achieved with active oxygen or chlorine. It’s enough to recall hydrogen peroxide and various versions of “bleach”, which easily discolor tea, coffee, pomegranate juice and the like. Necessarily included in the composition of both powders for dishwashers and washing machines. Softer options are used for the fabric, so as not to dissolve the factory pattern on the clothes. The odor is eliminated by the same oxidizing agents, since the "smelling" molecules also react with these components.
Mechanical pollution generally does not require the use of household chemicals. Sufficiently high pressure jet and hot water during washing dishes.
Washing powder in the dishwasher
You might think the idea is a little strange, but the washing powder machine is almost identical to the powder for dishwashers. In order not to be unfounded, let's analyze the typical composition and purpose of each component. For a detailed description I want to thank avor from the forum www.chemport.ru .
Analysis of the components of two typical representatives, one is "environmentally friendly", the second is "children's".
Ecover: zeolites, sodium disilicate, C12-18 sodium sulfate, sodium carbonate, sodium bicarbonate, sodium sulfate, ethoxylated rapeseed oil fatty acid methyl esters, sodium polyaspartate, sodium cocoate, cellulose gum, methyl cellulose, magnesium sulfate.
“Aistenok”: 5% -15% natural fat based soaps, oxygen bleach, phosphates (in terms of P2O5), less than 5% nonionic surfactants, sodium silicate, polycarboxylates, optical brightener, foam regulator, phosphonates, aromatic additives.
- zeolites - solid insoluble substances adsorb some impurities on themselves (they can leave fine dust when poorly rinsed). Inert substances are used in medicine as enterosorbents.
- sodium disilicate (water glass) - a thickener, glue, granule-forming agent is obtained on the basis of sand and caustic soda, it is dangerous only at high pH.
- sodium silicate - also like sodium disilicate.
- sodium carbonate, sodium bicarbonate is a soda ash and drinking soda used to regulate pH and soften water - toxic only high pH.
- C12-18 sodium alkyl sulfate is a synthetic surfactant based on fatty alcohol (the source can be either vegetable or petroleum) and sulfuric acid. Used in shampoos, toxicity is low or none.
- ethoxylated rapeseed oil fatty acid methyl esters - synthetic non-ionic surfactants based on ethylene oxide and LC methyl esters of rapeseed oil, a semi-natural product, toxicity see alkyl sulfates.
- sodium polyasparaginate is an expensive supplement based on a natural amino acid, serves as a thickener, water softener, and contaminant stripper; it is non-toxic absorbed by the body like food.
- sodium cocoate is an analogue of ordinary soap, not only based on animal fatty acids, but based on coconut oil fatty acids.
- cellulose gum is a derivative of cellulose (wood). The purpose is the same as sodium polyaspartate, toxicity is low.
- methylcellulose - similarly, but used as a thickener.
- magnesium sulfate. - It is used in medicine for both intravenous and oral administration - magnesia. In powders - an inert filler.
- Soaps based on natural fat are common soap, but on fatty acids of animal origin, similar to sodium cocoate
- oxygen bleach - sodium perborate or percarbonate is similar in properties to peroxide, which girls bleach hair, decompose and kill microflora during washing, decomposition products are low toxic (borates) or practically non toxic (carbonates).
- phosphates (in terms of P2O5) - a water softener and a pH regulator, sodium tripolyphosphate, phosphates are used as food additives and are low toxic.
- less than 5% nonionic surfactants are compounds similar to ethoxylated esters of LC rapeseed oil, however, products derived from oil (similar to alkyl sulfates) can also be used as a hydrophobic component
- polycarboxylates - either carboxymethyl cellulose or derivatives of polyacrylic acid (used in diapers, in particular in cosmetic gels), the toxicity is low, the purpose is the same as polyaspartates and cellulose gum (the latter can be identical in general)
- optical bleach - a dye such as blue causes white linen to shine with white light due to fluorescence, the toxicity is average, but the dose in the powder is 10,000 - 100,000 times less than necessary in order to feel a simple malaise from the action of this dye on the body.
- antifoam, antifoam - as a rule, silicone-based oil (analogues used for prosthetics of the breast) if it is not in the composition, then the powder can not be used in drum washing machines, the toxicity is extremely low.
- phosphonates - water softeners, low toxicity
- aromatic additives are usually less toxic than women's perfumes.
Actually, all of the listed components are non-toxic and passed all conceivable tests, including tests for washability, teratogenic and oncogenic effects in experimental rats. If you do not eat it with spoons, then there will be no health problems. If poorly rinse and toxic substances were used in the manufacture of laundry detergents for clothing, consumers would already have contact dermatitis from wearing such things. I liked the wording from the same forum, describing the "technological complexity" of the production of such powders:
Well, to make a good car, you need a whole science, expensive equipment.
Need an army of qualified specialists.
And to fumble household chemicals of this kind, you need a barrel, an oar, a bazaar scale, two guest workers.
And a boss who can read, write and count to one hundred.
Now let's compare the composition of powders for dishwashers. Take a completely standard Finish powder.

Here is a similar composition of the very common Somat powder:
Non-inogenic surfactants, phosphates, phosphonates, soda, silicate, polymers, oxygen-based bleaching reagents, TAED, enzymes, corrosion inhibitors, dyes, perfumes, water.
Now take the washing powder. I tried the Eared Nanny and Biolan options.

The same with minor variations.
Biolan’s composition is exactly the same:
Phosphates (15% or more, but less than 30%), anionic surfactants (5% or more, but less than 15%), nonionic surfactants (less than 5%), enzymes, flavoring.
As you can see, there is nothing criminal in the roster. The substances used by different manufacturers vary only slightly. Of the "excess" components for a dishwasher - only optical brightener. But this fluorescent is present in microdoses and rinses off perfectly during rinsing. In the same "Biolan automatic" it is not at all. Of course, there will always be abnormal chemophobes, but we will leave them the right to wash everything with soap nuts and mustard powder. If there are strange people who are ready to pay for it, then why not take a multiple mark-up from them for the Eco-Organic product? By the way, “Eared Nanny” is pleasant with its faint smell, but essentially identical in composition to other powders of our manufacturer “Neva Cosmetics” - Lotus, Max, Normal Powder, Sarma. The only difference is in flavoring and marketing positioning.
Let's play the alchemist
Having picked up a minimally smelling and foaming powder for automatic washing machines, one could calm down. The cost savings are already very substantial. But we will not rest on our laurels and try to radically drop the cost of the final dishwashing detergent. I immediately apologize for the quality of part of the photos - I shot them on slippers, and the normal camera is now at work.

As the basis of the product, we will take well-known and very cheap baking soda for everyone. Baking soda itself is sodium bicarbonate - NaHCO 3 . In solution it has a slightly alkaline reaction and has detergent properties. But it is much more efficient to use the product of its thermal decomposition - washing soda, it is sodium carbonate - Na 2 CO 3. Sodium carbonate already has a sharply alkaline reaction in solution, about 11 pH. It reacts with fats, forming soap, and proteins hydrolyzes successfully. Getting sodium carbonate is very easy - just heat it above 60 degrees.

I recommend a pan / skillet and heating in the oven for an hour. As a result, the resulting powder loses about 25% in weight.
Be careful when handling sodium carbonate!The substance may cause burns to the mucous membranes, eyes and other surfaces of the body. Use gloves, do not eat, do not inhale, and be careful. Soda is not a terrible poison, but alkaline burns have not brought joy to anyone yet. Now it remains to combine 70% sodium carbonate and 30% washing powder in order to add a small amount of detergents, bleach and other components. By the way, as a result of dilution, we reduce the total amount of potentially unwashed surfactants.
Preliminary tests

The first thing you will have to face with washing soda is fat contamination. As a test sample, a pan was taken after frying a pork chop.

After a standard cycle, the picture is more than acceptable. The pan is clean to the touch, I did not notice any greasy contaminants.

A particularly difficult test. Protein-fat contamination. Burnt oatmeal porridge in milk. Such contaminants are rarely taken the first time, even "special" powders. We try in enhanced mode, without first clearing anything.

The result is good. There were small traces of this disgrace, but I do not think that other powders would be washed better.
We consider savings
It’s a little difficult for me to calculate the exact figure, but try to at least roughly compare. The price of a conditional pack of soda weighing 500 g is 20 rubles. The price for 100 g is 4 rubles. You can find it much cheaper if you buy a bag in bulk, but it's too much. Now the powder. I would advise choosing powders with a minimal smell. "Eared nannies" for automatic machines is quite suitable. The price for packing 4.5 kilograms in the first available store is 439 rubles. The price for 100 g is about 10 rubles. In total, a kilogram of our product will cost 7 * 4 + 3 * 10 = 58 rubles.
Now the “special” Finish powder is 560 rubles per kg. The difference is about 10 times! If you take pills that contain the same, only with beautiful granules, then the difference will be absolutely beyond the limit.
Let's try to estimate the scale of savings for the year. About 20 g of powder is consumed per wash. In my family of 4 people, the dishwasher usually starts twice a day, but take 1 launch per day as the average. 20 * 365 = 7300 g of powder per year. This is 4088 rubles for the “special” and 423 rubles for our option.
Special salt

Another classic way of earning is a special salt for regeneration of the ion-exchange filter. Of course, rock salt and sand cannot be poured there. But let's raise GOST R 51574-2000 to table salt of the Extra grade . Mass fraction of NaCl - not less than 99.7%. The rest is minor admixtures of calcium and magnesium. Sorry, but this, according to the classification of the purity of chemical reagents, is better than “Pure for analysis” - “analytical grade”. This “Chemically pure” (“pure analytical grade”) is the highest degree of purity of the reagent. The content of the main component is more than 99%. The only caveat is that you do not need to take iodized or fluorinated varieties. Do you believe that salt, which is standardized by GOST for purity, is suitable for use in food, but not clean enough for a dishwasher?
Potential cons and problems
I want to clarify right away that I am not responsible for the operation of your dishwasher and any potential health problems associated with eating, drinking, or other obscure use of experimental results.I just describe my experience and information received in this area. If there are professional chemists among you, I will be glad to hear your point of view. The only problem that I potentially see is that because of the high alkaline reaction of the solution, it is possible to damage especially delicate painted porcelain, wine glasses or something like that. But such things can generally be washed without the use of detergents. I still recommend testing everything carefully and weighing it. From the point of view of health problems, I do not see anything seditious. We use surfactants even in a lower concentration than in special powders. The basis is a harmless sodium carbonate, which is part of antacid preparations for the treatment of gastritis. Allergic reactions to the components of washing powder? If you wash their clothes and everything is fine, then no problems are foreseen. With a manual wash, you leave many times more residual detergent for washing dishes on plates and cups. And nothing critical happens.
In any case, I would like you to consciously understand what exactly you are doing in terms of household chemicals and why.
useful links
It seems to me that the opinion of professional chemists and technologists is much more significant than traditional protein-hysteria at forums devoted to getting rid of terrible chemistry and a complete transition to "eco", "bio", "organic" products from "natural" components. I hope these links will give many people the opportunity to reconsider their views and stop suffering from chemophobia.
- Dishwasher detergent
- Using dishwasher detergent
- The chemical composition of safe household chemicals.
- Fantastic laundry detergent Umvay ??
UPD
Members Guzzle and apakin offer cheaper version of the local Russian manufacturer. For sale in Auchan, called Sanit. Approximate price is 100 r / kg. 2 times more expensive than the considered option, but sharpened directly under the dishwasher.
Pack photos



UPD2
Powder was not very. Washes decently, but worse than soda with detergent. Leaves traces on dishes, it is not completely washed off the surface when rinsing. Standard mode. Probably, it will be possible to dilute soda until there is a clean wash.
UPD3
Here Genegineer advised me to pay attention to the large packing of salt in the washers for large regeneration systems. Packing 25 kg, price around 600 rubles, i.e. in the region of 25 rubles per kilo. Here is an example from Leroy :

Only registered users can participate in the survey. Please come in.
