Weak household radiation
In this article I want to share my research on the topic of weak radiation sources that can be found in everyday life. I will not consider any exotic type of uranium glass products , instruments with radioluminescent paint on a scale and ionization smoke detectors . It will be about the most ordinary dishes, building materials and food products, whose weak and non-hazardous radioactivity can be detected by a simple household dosimeter.
The topic of radiation interested me after reading an article about a Geiger keychain . As rightly noted in the comments KbRadar, the keychain is a hazard signaling device, and not a search device for comparing the background radiation power in different places. Therefore, I wanted to get a simple dosimeter-radiometer with a screen. I wrote to the Dadget and ordered a Defender SOEKS dosimeter for review . It turned out that the device was already discontinued , and I got the last available copy. Therefore, in the article I will not describe in detail this particular gadget, but only give the results of studies conducted with its help.
First of all, I wanted to check the accuracy of the instrument readings. For
In the same place, my dosimeter showed this:
Since one hundred x-rays correspond to one sievert , the readings almost converge.
My dosimeter uses the good old beta-radiation sensor SBM-20 manufactured by Electrokhimpribor.
This sensor is a Geiger-Muller counter.non-responsive to alpha and soft beta (these types of radiation do not penetrate its metal casing). Nevertheless, it is ten times more sensitive than the SBM-21 used in the above keychain due to its size.
Counter type | Working interval U, V | Plate slope, % / V | MED, max R / h | Sensitivity, imp / s at 1 μR / s | Diameter, D, mm; Length, L, mm |
|---|---|---|---|---|---|
SBM-20 | 350 - 475 | 0.1 | 0.1 (p / s) | 60 - 75 | D = 11 L = 108 |
SBM-21 | 350 - 475 | 0.15 | 0.25 (p / s) | 6.5 - 9.5 | D = 6 L = 21 |
From this table ( taken from the site of Elektrokhimpribor ) it can be seen that SBM-20 will detect at least 15 pulses per minute with a natural background of 15 μR / h and SBM-21 - only 1-2 pulses. In a few minutes of measurements with SBM-20, you can gather enough statistics to show a more or less reliable value of a weak radiation background.
Potassium 40
One of the naturally occurring potassium isotopes, 40 K , is radioactive. Since it is chemically indistinguishable from ordinary stable potassium, along with stable potassium, it participates in the metabolism in living organisms and is part of many minerals. Every second, several thousand beta decays of 40 K occur in your body :
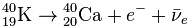
In addition, with a probability of 12%, a 40 K nucleus can capture an electron and turn into 40 Ar with the emission of a γ quantum.

The potassium-argon method of nuclear geochronology is based on this reaction .
Wood ash contains potash (potassium carbonate, K 2 CO 3) In the photo below, the counter lies in a bucket of ash left over from the preparation of barbecue. To make the difference with the natural background 0.12 μSv / h more noticeable, the dosimeter had to be buried in ash.
Note: If the goal is to obtain an accurate numerical value of the background, the dosimeter should not be kept in the immediate vicinity of the studied subject. In my case, the task was different - to detect the very fact of the presence of a small additional background.
The ash from burning grass contains more potash than wood, with it the differences would be more noticeable. Summer residents often use ash instead of factory-made potash fertilizers, which also fontain due to the presence of the 40 K isotope .
In the manufacture of crystal glass, the same potash or potassium oxide can be added to the mixture. Therefore, you can find weakly radioactive crystal glassware. I tried a bunch of vases and wine glasses and only inside this beer mug I noticed slight deviations from the background.
It is worth noting that measuring the radioactivity of objects makes sense only if you also measure the natural background nearby and look at the difference. Here you can see that the background is smaller from the mug.
A lot of potassium is found in bananas. Banana is even used as a comic radiation dose unit (see banana equivalent ). The background difference inside the box with bananas and a meter away is very small, but still detected.
In order to reliably detect such small differences in the background, you have to spend quite a lot of time on the measurements, because the SBM-20 error can reach 30%. The dosimeter updates the display every ten seconds. During each measurement, the green bar on the left side of the screen is filled. With each new measurement, the average value of all previous changes is displayed and thus the accuracy is increased. To indicate accuracy, there is a yellow bar that grows with each measurement and is completely filled in two minutes - the instruction says that sufficient accuracy is achieved with its maximum filling. In order to react to changes in the background, the logic of the device contains a reset of previous measurements when the background changes three times. In my experiments, there has never been such a significant difference,
To reliably fix the presence of a difference in background, I repeated the experiment with bananas several times. In each approach, I measured two background values - inside the box and next to the meter. Naturally, the numbers were floating a little, but in the box with bananas the background was always a bit higher.
Uranus and Thorium
These elements are primarily remembered when they talk about natural sources of radiation. Natural granite can contain both traces of uranium and thorium, although their amount is highly dependent on the deposit. In the park I found just such a decorative granite cobblestone, the background at the surface of which is twice as high as the background a couple of meters away.
Granite tiles, going to the cladding of buildings and monuments, can also fonit. I had to get around a lot of candidates until I found a twofold deviation from the background, which at that time was 0.12 µSv / h:
In construction, granite crushed stone is used that can be added to concrete or sprinkled on the road. Granite crushed stone is also used in railway embankments. In the photo below - Novomoskovsk Children's Railway(narrow gauge used to train young railway workers). Here, the rubble is good, not at all fonit.
Also in construction can be used slag - a byproduct of blast furnace steel production. Such weak-phased cinder blocks were popular among Soviet summer residents:
Where did uranium come from in slag? It is found in coal, which is burned in a blast furnace. Therefore, metallurgical plants and thermal power plants not only increase the level of carbon dioxide in the atmosphere, but also create radioactive contamination. Living near a thermal power plant can be more harmful than living near a nuclear power plant (as long as the latter is operating normally). Some of the uranium remains in the slag from which they make such cheap crushed stone and sprinkle them with tracks.
The track is slightly fonit.
Natural isotopes of uranium and thorium emit only α-particles that cannot penetrate the counter body. The counter reacts to β-active products of their decay (see Radioactive Series ).
Radon
Radon is a radioactive inert gas, seven times heavier than air. It does not have stable isotopes, the longest-lived of them, 222 Rn , has a half-life of slightly less than four days. The natural reserves of decaying radon are continuously replenished due to the α-decay of radium in the earth's crust.
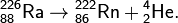
Due to their inertness, radon atoms easily leave the crystal lattice of the mineral in which they formed. Through cracks and pores, gas rises to the surface and enters the atmosphere, where it dissipates without causing much harm. Another thing is if radon does not go out into the open, but into the enclosed volume of the basement of the building. If the basement is not ventilated, radon will accumulate. SBM-20 cannot directly detect radon since this gas is subject to α-decay:
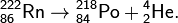
The nucleus of polonium 218 Po arising in this decay also decays with the radiation of an α-particle: 218 Po → 214 Pb + 4 He. But the lead nuclei 214 Pb are overloaded with neutrons and decay emitting β-radiation that "sees" SBM-20. There are other decay products (isotopes of polonium, bismuth, lead, etc.) emitting not only α but also β particles.
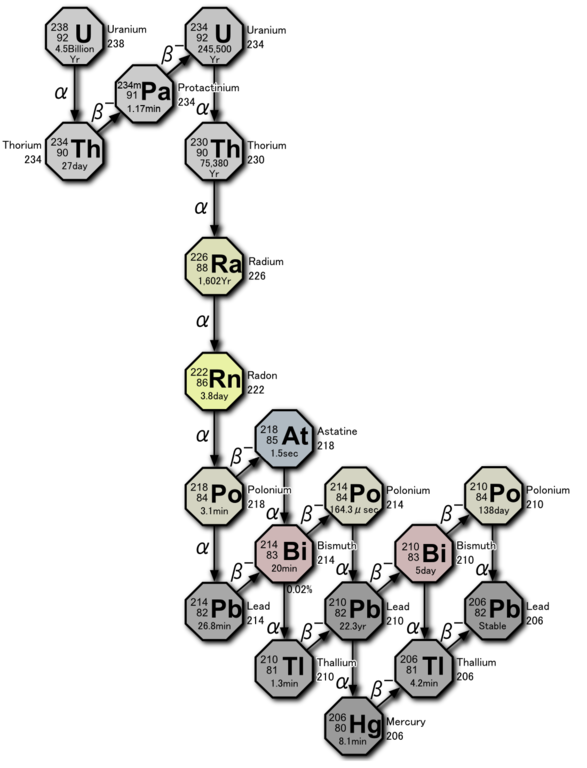
In general, special equipment is needed to accurately measure radon activity in air. With a domestic dosimeter, you can only try to detect the very fact of its presence. In search of radon, I went down to the basement of an old residential building with an earthen floor and measured the background at a height of one and a half meters (it amounted to 0.12 μSv / h). At the floor level, the background was only slightly higher, and I thought that there was no radon here, but I noticed that there was a large hole about a meter deep covered with boards in the floor (it was once used to store potatoes). I suggested that heavy gas can “drain” there through the gaps between the boards and accumulate, since the boards interfere with the ventilation of the pit. It turned out to be 0.3 μSv / h at the bottom of the pit.
I removed the boards, ventilated the basement and repeated the measurements:
The background has decreased markedly. It remains to try to explain the result. It would seem that there should not be any changes after airing, since the dosimeter does not respond to radon itself, but to the daughter products of its decay - heavy metals. Nevertheless, the experiment showed the presence of a difference in background. The diagram above shows that most of the formed metal isotopes lives minutes and seconds, and simply does not have time to settle on the floor. The atoms of daughter decay products condense on the smallest specks of dust hanging in the air, making them radioactive. Airing allows you to partially get rid of this dust.
Also, a little radon can get into our homes with natural gas and artesian water. Ventilate more often, because despite the fact that α-particles do not penetrate the skin, radon and its decay products enter the lungs when breathing. There they will no longer be so harmless.
Additional materials on the topic
In addition to the many links to Wikipedia articles that I posted in the text above, I can recommend the following interesting materials.
- Egigd article - A little bit about radiation
- Radiation at Lurkmore
- Dosimeter Test from Popular Mechanics
- An article in the journal Chemistry - Radioactivity in our home: the problem of radon
- The benefits and harms of radon
- Radiation Dose Chart on xkcd.com (there is also a Russian translation )
Update: When the article was already ready, news came from Dadget that a new model from the same manufacturer, Ecotexter 2 SOEKS, was on sale . This is a two-in-one combination device: dosimeter + nitrate meter. The device can measure the radiation background with the built-in Geiger counter SBM-20, and can also estimate the nitrate content in vegetables and fruits. In autumn, during a rich harvest, this is especially true. The functionality of the nitrate meter is based on measuring the electrical resistance of products with a probe. The company Dadget gives a 10% discount on Ecotexter 2 SOEKS to all readers of Gictimes. Promocode GEEKT-SOEX2 effect 14 days after the publication.
