Mercury
 The tendency of mercury to move from one form to another and the ability to cumulative accumulation is especially important in its technogenesis. In addition, mercury is omnipresent, sulfophilic, hydrophilic, multifaceted and is present in all environments and types of the environment, has many forms of finding, which makes it difficult to study. It is supertoxic and superpathological even in very low concentrations. Mercury is present in the lithosphere and biosphere in the form of solid compounds, various gaseous phases and in dissolved form, each of which prevails under specific physical and chemical conditions, but easily transform into each other. In technogenesis, mercury accumulates in the wastes of many industries, having high rates and destructive biological activity, it is able to produce hidden anthropogenic accumulations, but humanity cannot exist without this amazing metal. How mercury is monitored and controlled, what methods and instruments for its control exist - I propose to get acquainted under the cat.
The tendency of mercury to move from one form to another and the ability to cumulative accumulation is especially important in its technogenesis. In addition, mercury is omnipresent, sulfophilic, hydrophilic, multifaceted and is present in all environments and types of the environment, has many forms of finding, which makes it difficult to study. It is supertoxic and superpathological even in very low concentrations. Mercury is present in the lithosphere and biosphere in the form of solid compounds, various gaseous phases and in dissolved form, each of which prevails under specific physical and chemical conditions, but easily transform into each other. In technogenesis, mercury accumulates in the wastes of many industries, having high rates and destructive biological activity, it is able to produce hidden anthropogenic accumulations, but humanity cannot exist without this amazing metal. How mercury is monitored and controlled, what methods and instruments for its control exist - I propose to get acquainted under the cat.What is mercury produced from?
Take a look at this amazingly beautiful mineral that people have been interested in since ancient times. Until now, it is popular not only for its main purpose (to receive mercury), but I am for jewelers.

This is cinnabar - mercury (II) sulfide. Mineral for the production of mercury. Contains about 85 percent mercury, a brittle material with a characteristic red color. From ancient times, cinnabar was used as a red paint, as a source for mercury, and as the only reliable (albeit unsafe) treatment for infectious diseases that existed before the invention of antibiotics. As an indispensable bright scarlet mineral pigment, cinnabar was used already in Ancient Egypt and in early Byzantium. Everywhere since then, as in our days, natural cinnabar is widely used in canonical icon painting. But, of course, the most important use of this mineral is the industrial production of mercury.
Mercury is certainly an amazing material. This is the only metal that can exist in liquid form under normal conditions. It is a metal, therefore it is electrically conductive. But if the mercury is cooled to minus 39 g. C - it becomes solid and does not particularly differ from other metals. It can even be forged and sharpened. The network has an interesting video with a story about this wonderful substance. Mercury is used in a wide variety of technological processes, as well as in the production of gas discharge lamps, in microelectronics and instrument engineering. Mercury is an extremely technologically demanding substance and if mercury were not so toxic, its use would be even wider. I must say that mercury alone is not very dangerous - much more dangerous than its combination and vapor. So they are the sources of the main danger.
Mercury under control
Mercury can accumulate in soil, water, food, in humans and animals. Mercury in the form of vapors is always present in the surrounding air, but its “background” concentrations are not large. By the way, which ones? Rather stringent Russian standards for this case regulate the concentration of mercury in the air no more than 0.0003 mg / m 3 . Of course, registering and monitoring such concentrations is not an easy task and there are more than 25 registration methods for this.
Mercury Registration Methods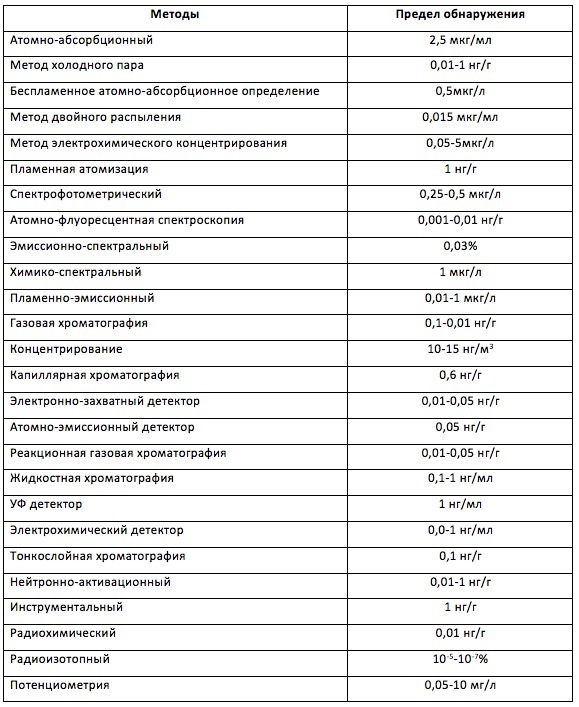

For example, chromatography. In this method, a separation process is carried out in which the test compound is distributed between the mobile phase (liquid or gas) and the stationary (solid or liquid). This method provides especially valuable information on the qualitative and quantitative content of inorganic and organic forms of mercury. When analyzing mercury in natural objects, it is possible to determine the halides of methyl, ethyl and phenyl mercury, as well as phenyl mercury, dimethyl and diethyl mercury, as well as some other less common organic forms of mercury. The disadvantage of this method of analysis is the technically sophisticated laboratory equipment and the method is used mainly to determine the mercury content in industrial and natural objects with a high mercury content, as well as in the soil.

There are a number of methods associated with the use of radioisotopes. Despite the formidable name, such methods are quite safe, since radioisotopes in negligible concentrations are used. To carry out the analysis, a precisely known amount of the determined component labeled with a radioactive isotope with known radioactivity is added to the test sample. After homogenizing the sample and undergoing isotope exchange, mercury is released from the medium (usually by chemical means) and its radioactivity is determined, which is then used to calculate the initial amount of mercury in the test medium.
 This method has a fairly high sensitivity, does not require expensive equipment and allows you to work with low concentrations of mercury.
This method has a fairly high sensitivity, does not require expensive equipment and allows you to work with low concentrations of mercury.Radio-indicator analysis methods allow solving such problems as determining trace amounts of mercury in substances, monitoring environmental pollution when analyzing the composition of atmospheric aerosols, loss of natural and waste water, and analyzing soils, as well as plant and animal objects. Radiation methods reliably guarantee the identification of mercury, have a sufficiently high sensitivity and can improve the accuracy and reproduced analysis results. In addition, such methods do not require expensive equipment, they allow working with a low level of radioactivity, which makes them indispensable for use in small laboratories, on research vessels, in conditions of high altitude stations, in expeditionary and field conditions. Method detection limit - up to 10 -6 - 10-8 %
If a good arsenal of control methods has been accumulated for determining mercury in liquid and solid media, everything is much more complicated to analyze the concentration of mercury vapor in the air. First of all, due to low concentrations of vapors in the air and due to the lack of fairly simple registration methods. The most promising is the registration method based on the Zeeman method . Let's consider it in more detail.
Mercury in the air
The Zeeman effect is the splitting of the lines of atomic spectra in a strong magnetic field. Since any substance has its own spectrum, if we use the spectrum of a special mercury lamp, but in the presence of a strong magnetic field, such a spectrum will be distorted. Additional components will appear in the spectrum, which will mirror the main spectrum. It looks something like this

The initial spectrum (black curve) when the magnetic field is turned on is distorted by three. The central spectrum (blue) and two symmetrical side spectra (shown in red). The magnetic field induction in this case is 1.56 T. This effect fundamentally allows you to implement a convenient method for registering mercury. For this, it is necessary to analyze the change in the amplitudes of the separated and the main components, and the higher the concentration of mercury in the air under study, the higher one of the components of the split spectrum and, at the same time, the smaller the other. Of course, air also has its own absorption spectrum at a wavelength of 254 nm (it is at this wavelength that a mercury lamp shines). This (in this case, "spurious") spectrum must be removed. For this, a “reference” channel and special filters are used.

The reference channel either does not contain mercury at all, i.e. demercurized , or there is exactly the known value of the concentration of mercury in the form of a reference. The radiation from a split-spectrum mercury lamp passes through a reference and measuring cuvette and enters a filter that filters the spurious spectra of other air molecules at a wavelength of 254 nm and enters the spectrometer. After the spectrometer, the reference and studied spectra arrive at the matrix, which is often subjected to cooling in order to increase sensitivity and for temperature stabilization. The resulting spectra are analyzed and the concentration of mercury in the test air is finally determined.
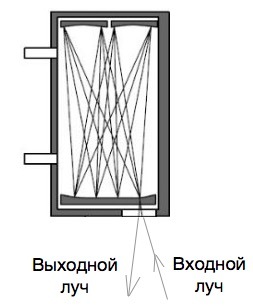 Of course, this is the most general representation of the circuit of such a device, in fact, because of the extremely low concentration of mercury in the sample, it is necessary that the optical radiation travels along an extended path in the measuring cell, for which various optical schemes for multiple transmission of optical radiation are used. This is done in order to maintain a relatively small size of the device to achieve a significant increase in sensitivity due to the multiple passage of the light beam. This, in turn, significantly complicates and increases the cost of the design due to the need for "fine" tuning of the device. The use of multi-pass ditches toughens the requirements for vibration, and there are already significant effects of temperature differences. However, these deficiencies are offset by a significant increase in sensitivity, after all, a beam in a multi-pass cuvette can “run” a significant path. Sometimes tens of meters. Most modern instruments use multipass cuvettes.
Of course, this is the most general representation of the circuit of such a device, in fact, because of the extremely low concentration of mercury in the sample, it is necessary that the optical radiation travels along an extended path in the measuring cell, for which various optical schemes for multiple transmission of optical radiation are used. This is done in order to maintain a relatively small size of the device to achieve a significant increase in sensitivity due to the multiple passage of the light beam. This, in turn, significantly complicates and increases the cost of the design due to the need for "fine" tuning of the device. The use of multi-pass ditches toughens the requirements for vibration, and there are already significant effects of temperature differences. However, these deficiencies are offset by a significant increase in sensitivity, after all, a beam in a multi-pass cuvette can “run” a significant path. Sometimes tens of meters. Most modern instruments use multipass cuvettes.
Mercury convention
Despite the unconditional demand for mercury for modern technologies, issues of a sharp reduction in its use in the near future are being considered. In 2013, the UN adopted a rather tough and very controversial Minamata Convention on Mercury , which was supported by many countries. According to the convention, the use of mercury should be regulated, and the production of certain mercury-containing devices (medical, fluorescent lamps) reduced. A number of industrial processes and industries are also limited, including mining (especially gold mining) and cement production.

Since 2020, the convention has prohibited the manufacture, export, and import of several different types of mercury-containing products, including electric batteries, electric switches and relays, some types of compact fluorescent lamps, fluorescent lamps with a cold cathode or with an external electrode, mercury thermometers, and pressure measuring instruments.
The initiators of the convention explain the intention to seriously limit the use of mercury in order to enhance the development of modern technologies in conditions where it will be impossible to use mercury and thereby significantly improve the environmental situation. However, some critics of the convention are of the opinion that this is just an excuse to review the global markets for mercury producers and force many players out of this market. After all, with the entry into force of the convention in 2020, the price of this metal can suddenly increase significantly, because humanity cannot refuse to use mercury completely.
Have a nice day, everyone!
