Planned first allotopic expression tests on mice.
- Transfer
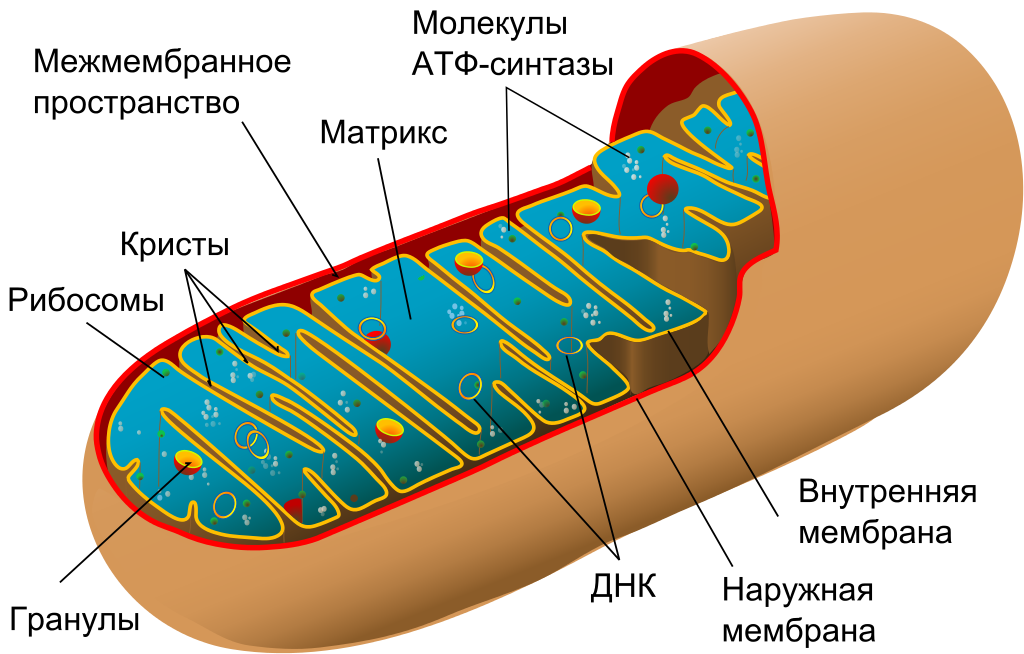
Mitochondria are the “energy stations” of the cell, descendants of ancient symbiotic bacteria. They retained a small fragment of the bacterial genome encoding thirteen genes necessary for the functioning of mitochondria. Most of the other genes passed into the cell nucleus during the process of evolution, as mitochondria became more and more integrated into the cell. Unfortunately, mitochondrial DNA is more susceptible to damage than nuclear, and some forms of damage can lead to mutations and malfunction of mitochondria. Mutant mitochondria quickly capture a cell, displacing their functional versions in the process of clonal expansion. Then this cell becomes an exporter of free radicals, which leads to a number of age-related pathologies. Peroxide lipids , for example, causeatherosclerosis .
The goal of the MitoSENS research program is to create backups of all mitochondrial genes in the cell nucleus using gene therapy , a process known as allotopic expression. It will in principle prevent changes in mitochondria by providing a backup source of proteins necessary for their proper operation. Of course, this is easier said than done. Genes need to be changed so that proteins can migrate into mitochondria, and insertion and migration optimization is a difficult task. Alas, due to the low level of funding, work has progressed slowly over the years that have passed since allotopic expression was first shown .
MitoSENS Campaign on Lifespan.io
Hello! This time we have two news. First, we are preparing a story about a new trick, which we used to improve the allotopic expression of mitochondrial genes. We are still studying whether we are 100% right before writing a publication and making a statement, but we are very close. Yes, this means that we have performed the allotopic expression of a larger number of genes. Be in touch!
Secondly, we are at the planning stage of our first mouse test, and we ask you to help in its launch! It will include the testing of two technologies that the SENS Research Foundation has helped to invent: the unique technology of transgenic mice and the application of what we learned in 2016year Our work, we are sure, will show the world that allotopic expression is real, and the future lies with it. In the near future you will see the announcement of a new test.
Matthew "Okey" O'Connor, PhD, head of the MitoSENS program
Interview with Matthew O'Connor on Longecity
Record in mp3
This week we tell you about the work of the SENS Research Foundation, which deals with the elimination of molecular and cellular damage. Aging of mitochondria is an important part of human aging. The SENS program that solves this problem, MitoSENS, is one of the most ambitious and technically challenging bioengineering projects. Recently, important events have occurred, and you will learn about them in our interview with the head of the MitoSENS program, Dr. Matthew O'Connor.
Longecity : You're on the air!
Matthew O'Connor : Hi, thanks for the invitation!
Longecity : Could you tell us a little about MitoSENS?
Matthew O'Connor: Of course. We are developing gene therapy that corrects mitochondrial mutations. The idea is that mitochondria have their own DNA, their own genes, a total of 13 genes encoding proteins, but all of them are important. Problems begin when mutations arise in them, either inherited from your mother or age.
Longecity : And age mutations affect almost everyone, right?
Matthew O'Connor : Right. We are not yet 100% sure, but everything indicates that the mitochondrial function decreases with age , and that this is an important aspect of aging that everyone experiences on himself, for example, in his muscles, as they become weaker with age.
Longecity: In our last meeting, you had only the concept of moving mitochondrial genes into the nucleus. The concept of MitoSENS was to transfer some of these genes to the nucleus of the cell, they would be better protected in it, and would continue to do their work. Anything new? Have you switched from the first two genes that you are targeting?
Matthew O'Connor : Mitochondrial DNA is more susceptible to damage because mitochondria specialize in creating energy, not in protecting and storing DNA. This is the task of the nucleus in which all our chromosomes live . Mitochondria produce energy and free radicals are a by-product of energy production.destroying sensitive DNA. Therefore, we tried to insert a backup of any of the thirteen genes in the nucleus. You mentioned two that we worked on. We had a publication at the end of 2016 in which we clearly showed that we can take a cell from a patient with mutations in two of the thirteen genes and correct them using our gene therapy.
Longecity : So, you were able to correct mitochondrial mutations, return mitochondrial functions in these cells. It sounds very good!
Matthew O'Connor: Yes, everything was very clear. We were able to show an increase in energy production by mitochondria, we were able to show oxygen consumption. The reason we breathe, consume oxygen, is because our mitochondria need it to produce energy. We were able to show an improvement in their survival. We were able to grow cells in two different conditions: anaerobically, as cancer cells usually do or how bacteria grow and in conditions where they could only survive aerobically, if they could consume oxygen using mitochondria. In aerobic conditions, only the corrected cells survived, and all mutant cells died.
Longecity : So you had success with these two first genes that you targeted. What about the other 11 genes? Any plans to work with any of them in the near future?
Matthew O'Connor : Yes, in fact, we are already working on all of them in varying degrees, and I can tell you a little about success. We developed targeting DNA vectors for all 13 genes coding for proteins, and we tested them for the ability to produce proteins and direct them to mitochondria. Not all of them work well, and we still can not declare victory. But we are making progress, and we will report on it soon. We show which ones work best and which ones work worse. We will talk about the strategies we are working on, with the goal of improving the continuous process of gene development and targeting mitochondria.
Longecity : I'm not a bioengineer, so could you clarify the mechanism by which proteins end up in mitochondria? How does this happen?
Matthew O'Connor: Mitochondria encode only 13 proteins, the nucleus encodes more than a thousand proteins that are transported into mitochondria. So their transportation is a rather ordinary process, and an unusual part is protein synthesis in mitochondria. We studied how the nucleus normally works, and we are trying to change the mitochondrial proteins so that they behave like nuclear proteins. Two simplest problems, for example, mitochondrial DNA is written in a slightly different language. She still uses the same four letters: A, T, G, and C , but the way they are read is slightly different. The first thing we need to do is translate the genes into the core language. The second thing we need to do is put the target sequenceat the beginning of the gene, called the mitochondrial targeting sequence or MTS. We take MTS from another gene and insert it at the beginning of any of our 13 genes in order to target the expressed protein to mitochoria. And we checked a lot of MTS in our lab.
Longecity : It sounds pretty complicated technically. You have been working on this for several years, which is the main problem in speeding up this potential anti-aging therapy?
Matthew O'Connor: So, the two things I just described are the relatively easy part, and the difficult part is optimizing the way the code works with MTS and other regulatory sequences that surround the gene, how the gene enters the genome, how many times it is inserted. Many different aspects that we study, they are the hard part, including our understanding of how evolution created this system, and figuring out how we can apply it to mitochondrial genes. We are constantly designing and redesigning, trying different changes in the genes to try to figure out how to improve their expression, targeting proteins to mitochondria, and then import into mitochondria, measure their function.
Longecity : People who follow rejuvenation research, know that all this is slow, tedious and difficult. Are there any new more effective tools?
Matthew O'Connor : There are two tools that help us. One of them is that in the current era of synthetic biology, you can order just a couple thousand dollars any DNA sequence from scratch. Therefore, in our time, unlike the time when I was in graduate school, we can simply type on the computer the code we want to create and synthesize it. In the old days, to create a new version, we used many different hacks, which took weeks and months, but nowadays we just need to print it and send it by email. It was a great blessing for us and our ability to test new ideas. Second -CRISPR , it is not new in molecular biology , but new in our project, it allows us to control where we insert our genes in the nuclear genome. He removes the extra variability that engineers had previously had to take into account when trying to insert his gene into the genome. This usually happens by chance, in any place, and this is an aspect that can complicate the situation. Nowadays, we begin to control the process by inserting genes more specifically with the help of CRISPR.
LongecityA: Nowadays there are many companies, they are looking at one of the aspects of aging, and it seems that suddenly there are some obstacles or unexpected things in their way. I know that you were very careful in planning how MitoSENS will develop. For several years, what was most surprising, or what sudden problems arose?
Matthew O'Connor: One of the problems is that the models of mitochondrial mutations are very limited. For example, I talked about using CRISPR for specific gene changes in the nucleus. But if you want to change genes in mitochondria, you cannot use CRISPR, it does not work in it, or at least no one has figured out how to make it work. Thus, it is impossible to manipulate the mitochondrial genome, which means that no one can create specific mutations in the mitochondrial DNA. We use random mutations that occur naturally. In addition, in model systems — such as mice, for example — there are not many mutations that are usually studied in the laboratory. There are very few mouse mitochondrial diseases, and therefore most of us use humans. This does not mean that we experiment with people we use human cells. We are limited to cells that are taken from patients who have found rare mitochondrial mutations. And our group is picky about the mutations we want to study, because we want specific mutations that affect only one, maybe two genes at a time, so that we can ask simple questions. Trying to do everything at once is not the best way. I would say that one of the biggest obstacles that slows us down is the lack of good cell lines for work. We are always looking for them - in publications and at conferences. so we can ask simple questions. Trying to do everything at once is not the best way. I would say that one of the biggest obstacles that slows us down is the lack of good cell lines for work. We are always looking for them - in publications and at conferences. so we can ask simple questions. Trying to do everything at once is not the best way. I would say that one of the biggest obstacles that slows us down is the lack of good cell lines for work. We are always looking for them - in publications and at conferences.
Longecity : And the next question is: when do you plan to work with whole organisms, and not just with the cells in the cup?
Matthew O'Connor : Good question, and I have an encouraging answer. We are planning to start fundraising for mouse trials in the coming months. We write financial plans. Labs promised to grow us transgenicmice. We completely designed the mice we need. We found mice with the desired mutations. They are not as important as those with which we usually work in cell lines, but with other mice they would not have survived, since mitochondrial mutations are very harmful to health. But we have mice with moderate mutations, and we have already done experiments on their cells, and they are considered to be working. Therefore, I think that we will have mice soon, but only after a couple of years we will find out if we have fixed the mutation. However, we will have mice with our gene, probably in less than a year.
Longecity : Sounds great. And the last question: you work with damaged mitochondria, and SENS aging theory says, hey, let's just fix the damageand everything will be much better. Do you have any thoughts on current products? Antioxidants like MitoQ or NAD + precursors , what do you think of them? Do you find them effective?
Matthew O'Connor : This is a difficult question, because it is not my field, but I will tell you my opinion. I would say that there are some preliminary studies indicating that increasing NAD + levels with the help of these dietary supplements can actually have some positive effect on your mitochondrial function. Whether they will improve your health or longevity is an unresolved question. But they could slightly improve energy production. Antioxidants aimed at mitochondriaare supposedly workers, but I do not recommend you run after them to the shops. And yet I believe that this area of research should pay attention. The era in which everyone talked about taking megadoses of vitamin C and E to try to remove all of the free radicals produced by mitochondria, is gone, as they do not get into your mitochondria. But some seem to fall into them. The problem is that this is a sensitive system, and it is better not to interfere in it. There have been experiments that have shown that some of these targeted antioxidants can remove free radicals unnecessarily well and actually damage the mitochondrial function. Therefore, I do not run to the stores for them, but follow the research.
