The energy in the cell. Use and storage

Hello! I wanted to devote this article to the cell nucleus and DNA. But before that you need to touch on how the cell stores and uses energy (thanks spidgorny ). We will touch on energy-related issues almost everywhere. Let's figure them out in advance.
What can you get energy from? Yes of all! Plants use light energy. Some bacteria, too. That is, organic substances are synthesized from inorganic due to light energy. + There are chemotrophs. They synthesize organic matter from inorganic due to the oxidation energy of ammonia, hydrogen sulfide and other substances. And there we are. We are heterotrophs. Who are they? These are those who do not know how to synthesize organic matter from inorganic. That is, chemosynthesis and photosynthesis, this is not for us. We take ready-made organics (eat). We disassemble it into pieces and either use it as a building material, or destroy it for energy.
What exactly can we disassemble for energy? Proteins (first breaking down them into amino acids), fats, carbohydrates and ethyl alcohol (but this is optional). That is, all of these substances can be used as energy sources. But for its storage we use fats and carbohydrates . I love carbohydrates! In our body, glycogen is the main storage carbohydrate.

It consists of glucose residues. That is, it is a long, branched chain consisting of identical units (glucose). If necessary, in energy, we split off one piece from the end of the chain and oxidizing it, we get energy. This method of generating energy is characteristic of all body cells, but especially a lot of glycogen in the cells of the liver and muscle tissue.
Now let's talk about fat. It is stored in special connective tissue cells. Their name is adipocytes. In fact, these are cells with a huge fat drop inside.
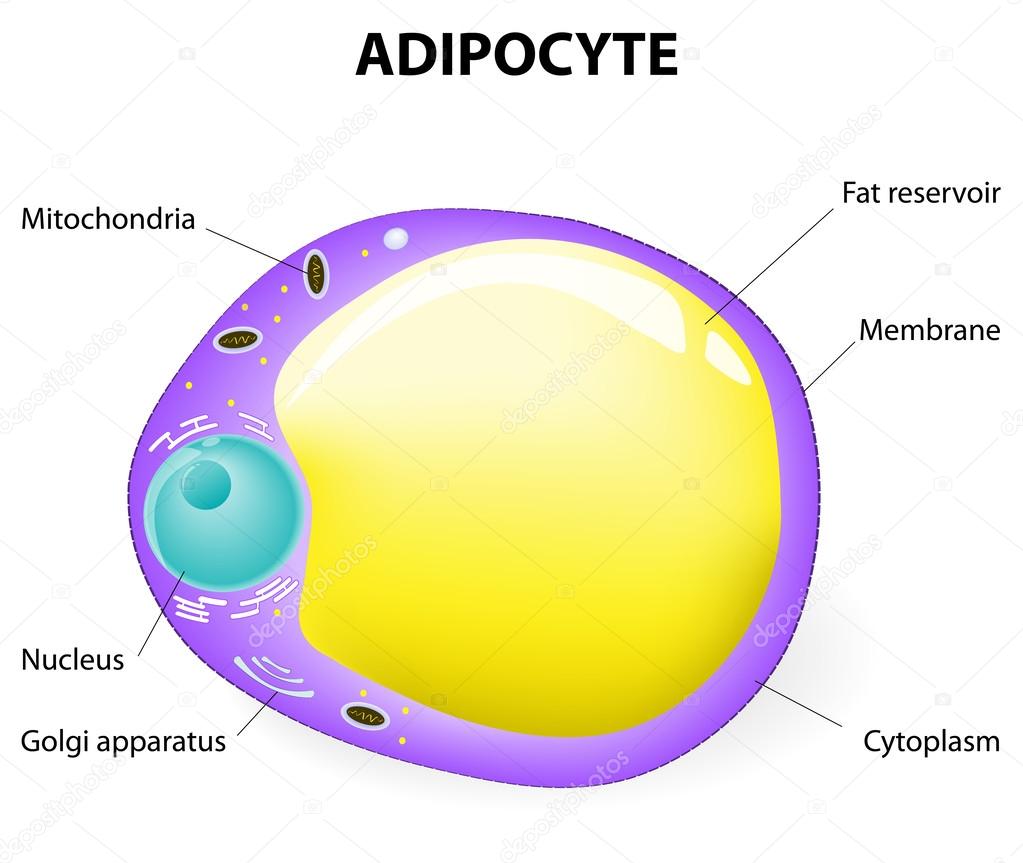
If necessary, the body removes fat from these cells, partially breaks down and transports. At the place of delivery, the final splitting occurs with the release and conversion of energy.
A rather popular question: “Why can not all energy be stored in the form of fat or glycogen?”
These energy sources have different purposes. From glycogen, energy can be obtained quite quickly. Its cleavage begins almost immediately after the start of muscle work, reaching a peak in 1-2 minutes. The breakdown of fats occurs several orders of magnitude slower. That is, if you sleep, or slowly go somewhere - you have a constant energy expenditure, and it can be achieved by breaking down fats. But as soon as you decide to accelerate (the server crashed, ran to lift), it will take a lot of energyand quickly get it by splitting fats will not work. Here we need glycogen.
There is another important difference. Glycogen binds a lot of water. Approximately 3 g of water per 1 g of glycogen. That is, for 1 kg of glycogen, this is 3 kg of water. Not optimal ... Fat is easier. Lipid molecules (fats = lipids) in which energy is stored are not charged, unlike water and glycogen molecules. Such molecules are called hydrophobic (literally, afraid of water). Molecules of water are polarized. It looks something like this.
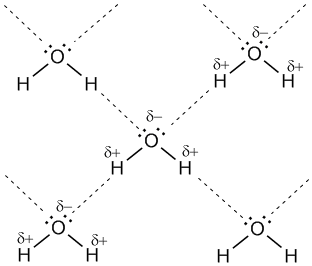
In fact, positively charged hydrogen atoms interact with negatively charged oxygen atoms. It turns out a stable and energetically favorable state.
Now imagine the lipid molecules. They are not charged and cannot interact normally with polarized water molecules. Therefore, a mixture of lipids with water is energetically disadvantageous. Lipid molecules are not able to adsorb water, as glycogen does. They are "grouped" into so-called lipid drops, surrounded by a membrane of phospholipids (one side is charged and facing the water from the outside, the other is not charged and looks at the lipids of the drop). As a result, we have a stable system that effectively stores lipids and nothing more.
Okay, we figured out what forms energy is stored in. What happens to her next? So we split the glucose molecule from glycogen. Turned it into energy. What does it mean?
Let's make a small digression.
About 1,000,000,000 reactions occur every second in the cell. When the reaction proceeds, one substance is transformed into another. What happens to his inner energy? It can decrease, increase or not change. If it decreases -> energy is released. If it increases -> you need to take energy from the outside. The body usually combines such reactions. That is, the energy released during the course of one reaction goes to the second.
So in the body there are special compounds, macroergs that are able to accumulate and transmit energy during the reaction. In their composition there is one, or several chemical bonds in which this energy accumulates. Now you can return to glucose. The energy released during its decay is stored in the bonds of these macroergs.
Let's take an example.
The most common macroerg (energy currency) of a cell is ATP (Adenosine Triphosphate).
It looks something like this.
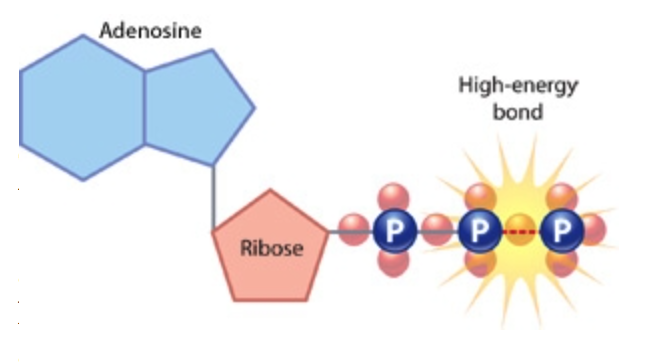
It consists of a nitrogenous base adenine (one of 4 used to encode information in DNA), ribose sugar and three phosphoric acid residues (and therefore Adenosine TRIPHOSPHATE). It is in the bonds between the residues of phosphoric acid that energy is accumulated. When one residue of phosphoric acid is cleaved, ADP (Adenosine Diphosphate) is formed. ADP can release energy, tearing off one more residue and turning into AMP (Adenosine MONO phosphate). But the efficiency of the split off second residue is much lower. Therefore, usually, the body seeks from ADP to get ATP again. It happens like this. With the breakdown of glucose, the energy released is spent on the formation of a bond between the two phosphoric acid residues and the formation of ATP. The process is multi-stage and so far we will omit it.

The resulting ATP is a universal source of energy. It is used everywhere, starting from protein synthesis (energy is needed to connect amino acids), ending with muscle work. Muscle contraction motor proteins use the energy stored in ATP to change their conformation. A change in conformation is a reorientation of one part of a large molecule relative to another. It looks something like this.
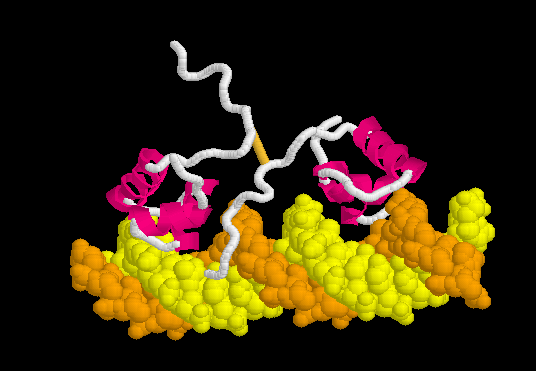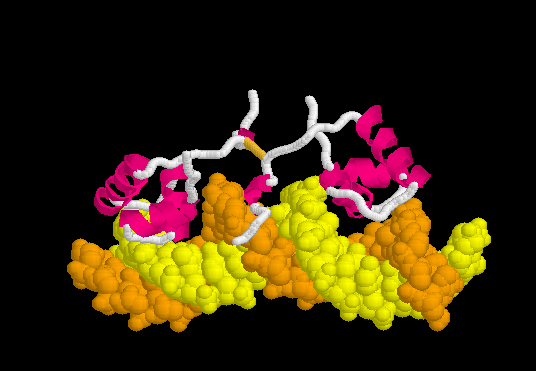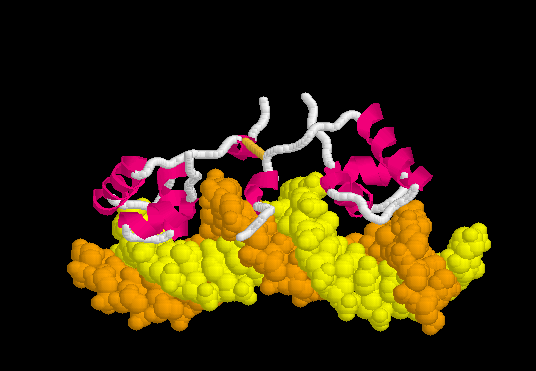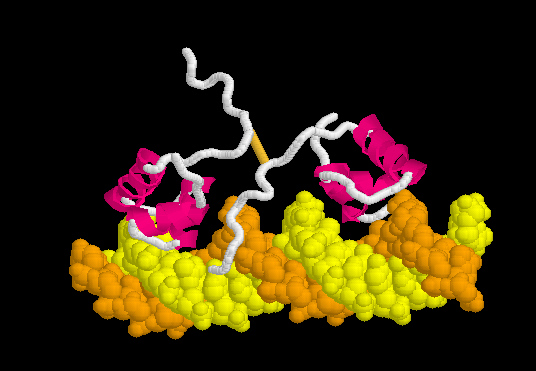
That is, the chemical binding energy transforms into mechanical energy. Here are real-life examples of proteins using ATP to do the job.
Meet this myosin . Motor protein. It carries out the movement of large intracellular formations and is involved in muscle contraction. Please note that he has two “legs”. Using the energy stored in 1 ATP molecule, he makes one conformational change, in fact one step. The most obvious example of the transition of chemical energy from ATP to mechanical.

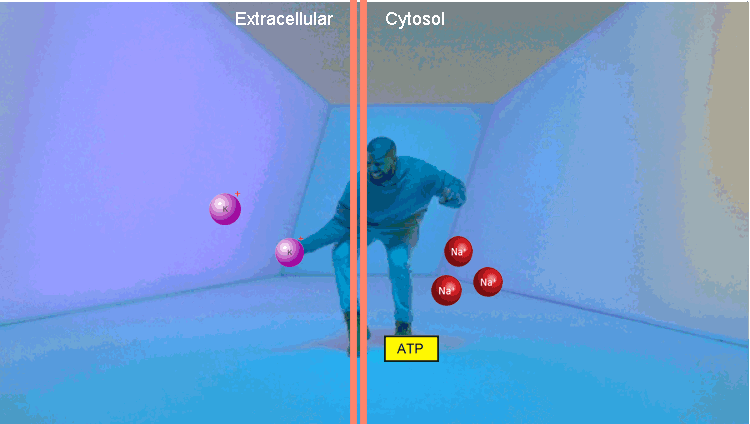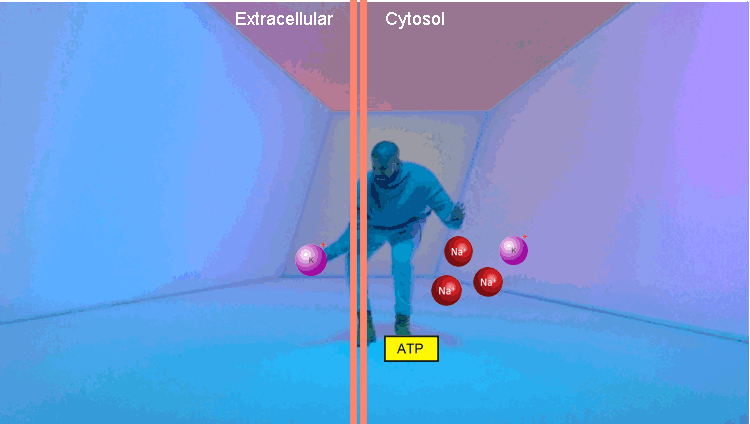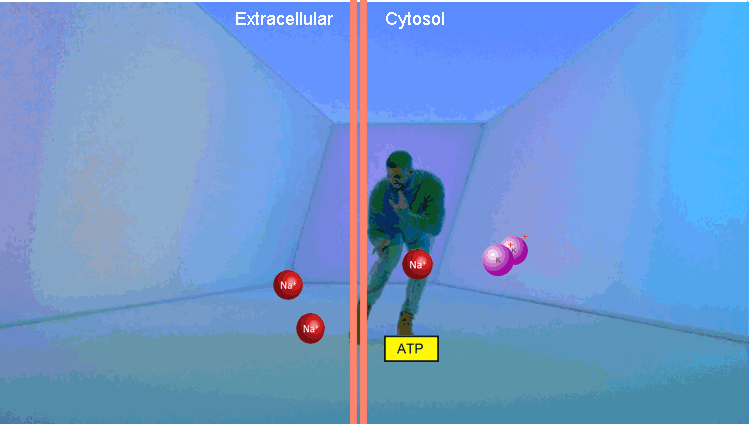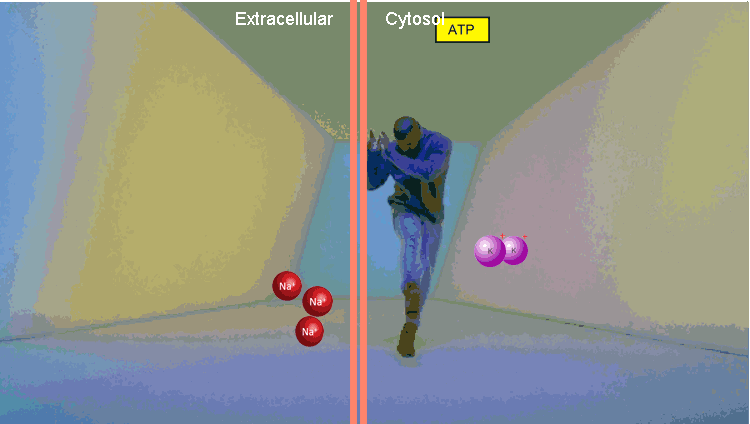
The second example is a Na / K pump. At the first stage, it binds three Na molecules and one ATP. Using the energy of ATP, he changes conformation, throwing Na out of the cell. Then it binds two molecules of potassium and, returning to the original conformation, transfers potassium to the cell. The thing is extremely important, it allows to maintain the level of intracellular Na in the norm.
But seriously, then:

Pause. Why do we need ATP? Why can't we use the energy stored in glucose directly? Trite, if you oxidize glucose to CO2 at a time, extremely much energy is instantly released. And most of it will dissipate in the form of heat. Therefore, the reaction is divided into stages. A little energy is released on each, it is stored, and the reaction continues until the substance is completely oxidized.
I will summarize. Energy is stored in fats and carbohydrates. It can be extracted faster from carbohydrates, but more can be stored in fats. For reactions, the cell uses high-energy compounds, which store the energy of the breakdown of fats, carbohydrates, etc. ... ATP is the main such compound in the cell. In essence, take and use. However, not the only one. But more on that later.
PS I tried to simplify the material as much as possible, so some inaccuracies appeared. I beg the zealous biologists to forgive me.
