SENS-Diagnosis. Protein glycation biomarkers
The development of aging therapy is impossible without a reliable diagnosis of aging. It does not seem reasonable to wait for the death of a person or the onset of a deadly disease, in order to understand: have we slowed down the aging or not of one or another therapy. We must immediately see an objective picture, caused by our interventions against aging.
In clinical practice, there is still no "big diagnosis of aging." That is, it is not possible for the patient and the attending physician to detect age-related changes at the molecular level prior to the onset of the disease. We want to eliminate this gap, first of all by describing all the main markers of age-related changes and the available technological level for their measurement.
We will continue to present the concept of SENS-diagnostics of aging, based on the fact that today the SENS program (the achievement of negligible aging by engineering methods) most fully describes the approaches to increase human longevity.
Many have heard about the glycosylation of proteins, the end products of glycation (CNG, AGE) and about the harm they cause to the body. But, it should be noted that the addition of sugars to other molecules is not always a pathology. Glycosylation itself is a very common and important physiological process in living organisms. Thus, a significant part of all proteins synthesized in cells undergo enzymatic glycosylation, which is necessary for their normal functioning.
Mainly glycosylation involves two glycans (carbohydrate portion of the carbohydrate-organic molecule ligament): N-glycans (linked to the amide group of asparagine) and O-glycans (linked to the hydroxyl group of serine or threonine). Due to aging, we are more interested in N-glycans. It is described that with aging, the spectrum of sugar chains changes, which are attached to immune proteins upon N-glycosylation. And such a change plays a key role in the age-related increase in total inflammation in the body. Thus, the level of glycosylated IgG antibodies can predict a person’s biological age even more accurately than telomere length [1].
In addition, two more glycans, NGA2F and N2AF, proved to be promising biomarkers of aging. In the framework of the European ––––– –––––––––––––––––––––– program, the GlycoAgeTest test, which determines the biological age of a person, was developed by the MARK – AGE European Biomarker Research Program, completed in 2013. It is based on the ratio of the number of glycans NGA2F (increasing with age) and N2AF (the level of which decreases with age). Another potential biomarker of aging and age-related diseases (cardiovascular and diabetes), described in the framework of the MARK – AGE study, was the glycoprotein clusterin, which is involved in the stabilization of protein structures [2].
We now turn to the consideration of the pathological part of this phenomenon. In addition to the enzymatic glycosylation regulated by the body, there is a non-enzymatic form of this process, the so-called. the Maillard reaction, which results in the appearance in the body of a variety of glycation products. It should be noted that the process of non-enzymatic glycosylation is practically not regulated. Although there is a possibility of "restraining" glycosylation through transglycation, in which glutathione, polyamines, thiols, free amino acids, for example, taurine, lysine, are used as "consumption". And also through the inactivation of methylglyoxal by the glyoxalase system: glyoxalase I converts methylglyoxal and reduced glutathione into lactoylglutathione, which is further metabolized to D-lactate by the action of glyoxalase II.
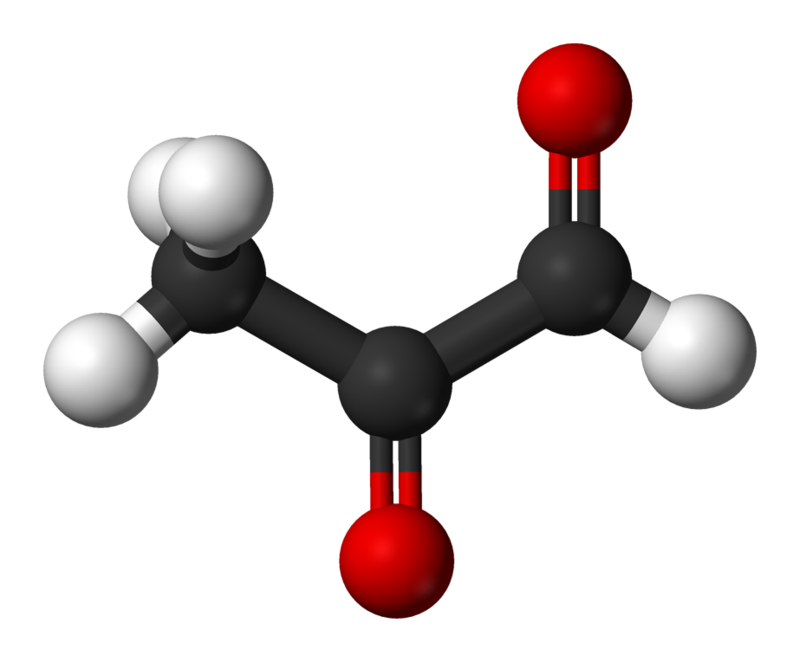
The structure of methylglyoxal
Early (products of Amadori) and late (or final) products of glycation are distinguished from the products of glycation. Nonenzymatic glycosylation occurs in several stages. This process begins with the fact that glucose and other simple sugars combine with the amino group and start a chain of further reactions. In the beginning, during the reaction between the aldehyde group of sugars and the amino group, an unstable aldimine group (Schiff bases) is formed, which can turn into a number of other, more stable compounds, early glycosylation products, the so-called. Amadori products. One of the early products of glucose attachment to a protein is Ne – fructosyl – lysine, which, in turn, degrades, forms various end products of glycation (CNG). Hydroimidazolones, derivatives of arginine, are formed in the greatest amount as CNG, modified with glyoxal, methylglyoxal and 3 – deoxyglucosone (3 – DG). Another well-studied CNG is Nδ – carboxymethyl – lysine (CML) and Nδ – carboxyethyl – lysine (CEL), as well as glucosepan and pentosidine, which are characteristic of protein cross-links [3]. One of the most easily identified types of CNG is pentozidine, which accumulates, for example, in the tendons of large human muscles (that is, where the rate of collagen turnover is slowed) since 20 years and its concentration increases linearly with old age.
Until recently, it was believed that glucose is the main substance for the formation of CNG. But the exact establishment of different rates of intra-and extracellular formation of CNG showed that this is not the case. Such sugars as fructose, glucose-6-phosphate and glyceraldehyde-3-phosphate have a higher rate of intracellular formation of CNG. The negative effect of glyceraldehyde on the molecule of the main contractile and cytoskeletal protein actin, leading to the formation of pentosidine and bityrosine crosslinks and loss of actin functionality, has been described [4].
Stitching collagen molecules with glucosean.
As suggested today, non-enzymatic glycosylation and CNG are closely associated with a number of age-related diseases, such as diabetes, rheumatoid arthritis, atherosclerosis, Parkinson's and Alzheimer's disease, amyotrophic lateral sclerosis, cataracts and oncological diseases [5]. Proteins subjected to glycation in the Maillard reaction, become aphysiologically cross-linked, losing their properties. Especially a big problem for long-lived proteins (for example, skin collagen molecules, according to several studies, have a half-life of 15 years, and cartilage is more than 100 years), which constitute a significant part of all proteins in the body - about one-third. Formed additional cross-links between molecules violate the functions of these proteins, which leads to a loss of tissue elasticity and is often observed during aging and pathologies.
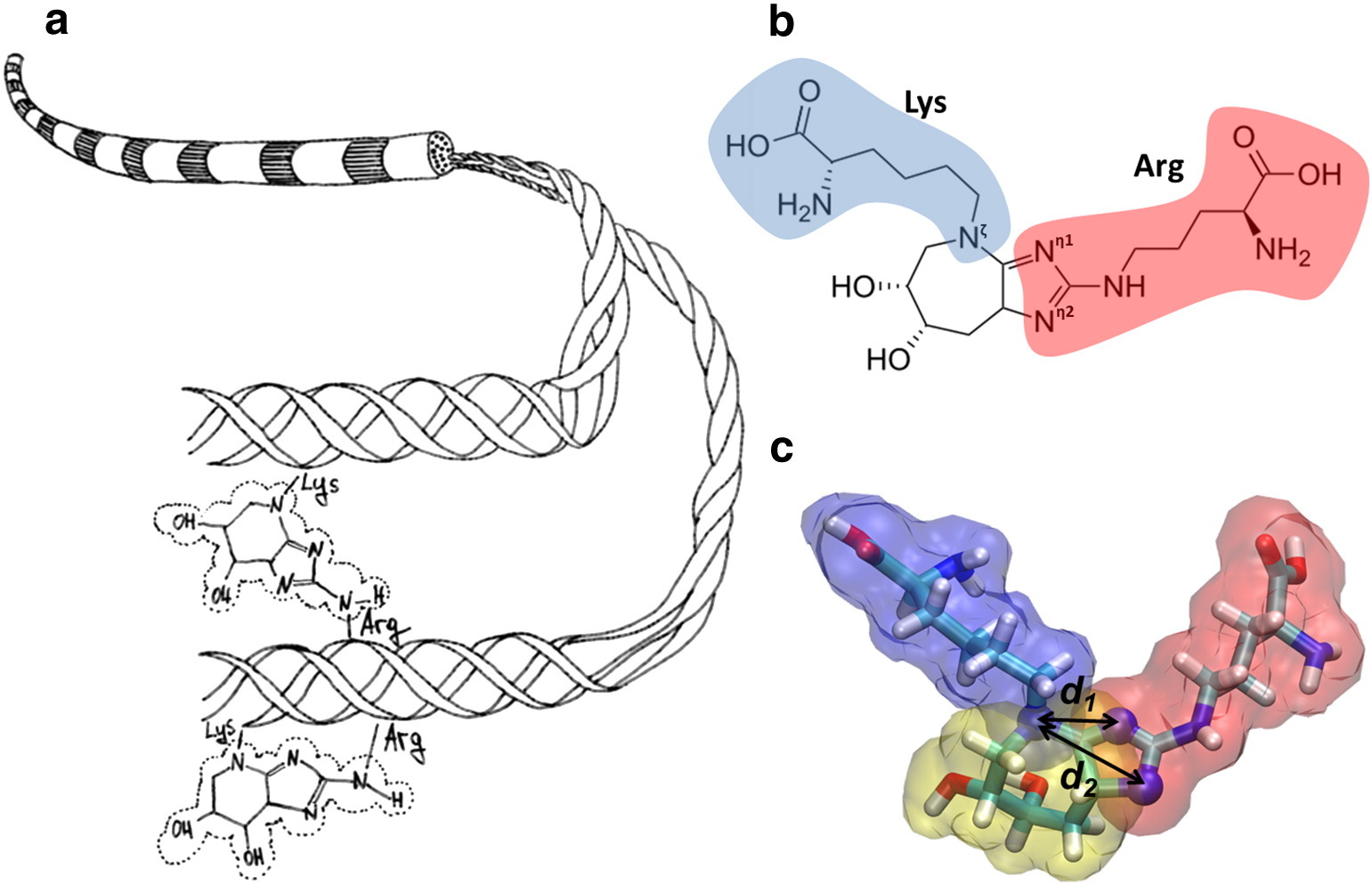
Illustration (a) shows a diagram of collagen fibril and the formation of glucoseepan, which covalently binds the side chains of lysine and arginine. (b) The chemical structure of glucosopan is shown, which crosslinks lysine (blue) with arginine (red). (c) A molecular model of glucose is shown.
The involvement of glycated fibroblast growth factor β-FGF in the formation of fibrosis has been described. Glycation of the arg-gly-asp protein of fibronectin leads to a violation of vascular restorative ability and progression of vascular pathologies. CNG accumulates in the lens and retina with age. Crystallins, the major structural proteins of the lens of the eye, are susceptible to glycation and cross-linking. Glycation of lens proteins leads to cataracts. Glycated hemoglobin, which is used to determine the average level of glycemia over the past 3 months, loses the ability to carry oxygen to tissues, which negatively affects many chemical processes.
It is also known that protein glycation and the formation of CNG is accompanied by an increase in free radical activity and lipid peroxidation, which contributes to the development of age-related diseases. This is due to the fact that the process of glycation of proteins triggers the auto-oxidation of sugars, accompanied by an increase in the production of reactive oxygen species (ROS). In addition, the binding of CNG to the CNG receptor (RAGE) also leads to the formation of reactive ROS and the subsequent activation of the oxidative stress-sensitive transcription factor NF-kB, associated with inflammation and atherogenesis.
In turn, activation of NF-κB increases RAGE expression, creating a positive feedback cycle that enhances the production of inflammatory promoters. In addition, the interaction of CNG-RAGE activates NADPH oxidase (a complex of enzymes that produces superoxide), which increases intracellular oxidative stress. And an increase in oxidative stress with NADPH oxidase in response to AGE-RAGE interaction activates, in turn, NF-κB. And here we can observe a whole series of “vicious circles” - cycles with positive feedback.
Glycation of the mitochondrial respiratory chain proteins also contributes to this process: a malfunction of the respiratory chain is a constant source of superoxide radicals. In addition, the formation in mitochondria and other structures under the influence of glycation of non-degradable CNG may be responsible for the implementation of such a phenomenon as "metabolic memory". When in patients with type 2 diabetes, strict glycemic control no longer prevents the development of complications associated with cardiovascular diseases. It is assumed that non-decomposable CNGs play a major role in this phenomenon [6].
Also during glycation, an increase in inflammatory processes occurs. So, during collagen glycosylation, accumulation in the extravascular matrix of the end products of this process, which the body perceives as foreign, occurs. Because of this, an immune response will occur, during which immune cells - macrophages - will increase the secretion of pro-inflammatory cytokines (TNF-α, interleukin-6, etc.), which are known for their ability to adversely affect insulin resistance and atherogenesis [7].
In addition, the “cross-linked” proteins change the structure (nanotopography) and increase the rigidity of the extracellular matrix and, through the mechanoreceptors of the membrane and the cytoskeleton of the cells, together with the signaling molecules, affect gene expression, the function of cells, tissues and the organism as a whole. Moreover, thanks to proline, collagen molecules conduct weak electromagnetic waves generated by cells and tissues, and, perhaps, in addition to mechanical and chemical signals, form a single bioelectric signal system of the body. It is interesting to note that by changing the topography of the medium or electromagnetic radiation, it is possible not only to control the cell cycle, but also to transform adult somatic cells into stem cells without the help of viruses with the vector of Yamanaka factors.
Even minor changes in the shear modulus and viscoelasticity of the extracellular environment have a strong effect on the cells — the “old” extracellular matrix can significantly limit the effectiveness of the use of senolysis and stem cell therapy. So, “young” fibroblasts rapidly grow old in the old matrix and vice versa - the “old” cells lose the signs of the secretory phenotype associated with aging in the “young” matrix.
What are the causes of glycated proteins and CNG? It is believed that the main one for modern man today is junk food. The roasted brown-black crust on the products represents the collection of CNG, which are formed due to the heat treatment of products (roasting meat, potatoes, etc.) at high temperatures. The record for the number of formed KKE is considered to be fried bacon. In addition, the consumption of foods containing a lot of fast carbohydrates (confectionery, sugary carbonated drinks, etc.) can also contribute to protein glycation and the formation of CNG. There are products that, on the contrary, help to resist the accumulation of CNG - black radish, radish, broccoli, due to the substance contained in them sulforaphane. As it is not surprising but along with the gourmets regularly supply themselves with higher doses of CNG and smokers. Tobacco smoke contains glycotoxins, which react with lysine and arginine residues to form CNG. This is probably why smokers have chronic inflammation of the respiratory organs [8].
As already mentioned, in addition to the influence of external factors, the formation of CNG in normal conditions also occurs endogenously (that is, inside the body) under physiological conditions. Although this process is slow and insignificant, it is significantly enhanced with an increased concentration of free radicals, insulin resistance, diabetes mellitus and an increase in blood glucose levels. Accumulating in the body, CNGs drag people into the “swamp” of pathological processes, from which it is not so easy to get out.
According to the figurative expression of SENS authors, protein crosslinks act as molecular “handcuffs” that bind protein molecules, disrupting their function. The solution to this problem, the authors of SENS see in the development of drugs that can react with cross-links and break them without destroying the other structural features of the molecules. In their opinion, a favorable circumstance in this process is that crosslinks, which occur as chemical accidents in the structures of our protein molecules, have a very unusual chemical structure, which normally does not occur in substances produced by the body. Which should facilitate the search and creation of therapeutic agents.
In addition, the authors of SENS define a number of promising approaches to solve this problem. Such as the search or development of enzymes, instead of drugs, to destroy crosslinks. As well as the development of "disposable" proteins that would destroy the cross-linking, and then they themselves would be destroyed in the process. It is known that such proteins exist for other purposes, for example, the DNA regenerating protein MGMT [9]. In favor of the approach to the search for enzymes, says the fact that living organisms described deglykiruyuschie enzymes. Thus, fungi and bacteria found anti-glycation enzymes amaadoria, fructozolysin-6-kinase frlD and fructosezolysin-6-phosphate-deglycase frlB, which act on low-molecular-weight amino acid compounds with sugars [10]. In vertebrates, the enzymes fructosamine-3-kinase (FN3K) and its related protein FN3K-RP are found.
It is believed that glukezepan has the greatest impact on the course of the diseases of the elderly person and therefore is a priority target for anti-aging therapy.
Unfortunately, glucoseepan was not targeted for previously developed anti-crosslinker drugs such as Alagebrium / ALT-711 (Wolffenbuttel et al., 1998), C36 (Cheng et al., 2007), TRC4149 (Pathal et al., 2008 ), and he only has to prove his role in the mechanisms of aging. These substances were aimed at neutralizing carboxymethyl lysine, the most common late product of the Maillard reaction, which accumulates in the body in diabetes.
Currently, a group of David Spiegel from Yale University is working on the synthesis of antibodies against glucoseepan-containing proteins. Aubrey de Gray confirms the importance of research on glukezepan and recently announced the registration of the company Revel, on the basis of which research initiated at the university may be continued.
However, it is likely that neither antibodies nor enzymes, due to their size, will be able to penetrate between collagen fibrils. Moreover, the broken cross-links of collagen are restored after the end of the drug intake, which will require a repeated course of therapy.
Therefore, artificial enzyme-like catalysts, the dimensions of which may be several times smaller than the sizes of the original enzymes, seem to be a more interesting alternative. Such molecules with a given catalytic activity — spioligomers (spiroligomeres) are being developed by the team of Christian Schaffmeister from Temple University.
As noted earlier, since the end products of glycation accumulate during aging, their quantitative determination can be an accurate and reliable biomarker of aging. However, both the detection of CNG in the body and the early diagnosis of changes in the composition and structure of the extracellular matrix are difficult due to the nature of the changes limited by organs and systems and the need for a multiple biopsy. Even in one organism, the results are very different depending on the place of biopsy and the type of glycation end products studied.
Available methods for determining the end products of glycation in the skin, such as autofluorescence, that is, without the use of special dyes (the “AGE-READER” device manufactured by the Dutch company DiagnOptics BV) do not provide accurate data and many factors can affect the measurement results - salt balance of the body.
The main and most accurate method for quantifying CNG in the body today is the method of chromatography combined with mass spectrometric detection. Mass spectrometry makes it possible to identify proteins with a high degree of reliability and to determine their amounts in complex protein mixtures.
An enzyme immunoassay is also used to determine the level of CNG. But this method has a number of qualitative limitations (insufficient specificity of antibodies, the effect of free glycation products, etc.). Therefore, it is considered that it is advisable to carry out it in combination with mass spectrometry. CNG can also be identified by the total intensity of their fluorescence. What also has its limitations (most of the CNG do not quantitatively fluoresce, therefore, cannot be determined) and allows you to more accurately determine the low molecular weight peptides of CNG and free glycation products.
One of the promising biomarkers, showing not only the accumulation of CNG, but also an increase in the risk of all-cause mortality, is plasma carboxymethyl-lysine (CML). KML is one of the dominant CNG in the body, both circulating and tissue. In addition, KML is the only CNG that acts as a ligand for CNG receptors (RAGEs). The binding of RAGE to KML leads to an increase in the generation of free radicals, activation of the pathway of the nuclear factor Nf-κB and an increase in the level of inflammatory mediators (such as tumor necrosis factor alpha, interleukin-6 and C-reactive protein). It is known that KML accumulates in the large blood vessels with age. And high concentrations of this CNG in serum are associated with greater arterial stiffness, a powerful risk factor for the development of cardiovascular pathologies and mortality from them,
It is also known that elderly people with cerebrovascular diseases, elevated levels of KML are found in cortical neurons and brain vessels, which is associated with the severity of cognitive impairment. As part of the 6-year study of Invecchiare in Chianti, which was attended by 1,013 people over 65, it was shown that the average plasma concentration of KML, which was measured by ELISA, was significantly higher in those who died of all causes than survivors [13]. In 2018, a group of Danish and Swedish scientists described a new monoclonal antibody, D1-B2, aimed at KML, which has good potential in ELISA to detect this CNG. [14]
Previously, using coupled liquid chromatography along with mass spectrometry, a number of CNG (glucosean, DOGDIC, MODIC and GODIC) were identified that accumulate in tissues during aging and pathology and can be used as markers for pathophysiological processes [15]. The method of high-performance liquid chromatography allows to detect two more classes of CNG associated with aging and diabetes - GOLD and MOLD [16].
Review authors: Denis Odinokov, Alexey Rzheshevsky.
In clinical practice, there is still no "big diagnosis of aging." That is, it is not possible for the patient and the attending physician to detect age-related changes at the molecular level prior to the onset of the disease. We want to eliminate this gap, first of all by describing all the main markers of age-related changes and the available technological level for their measurement.
We will continue to present the concept of SENS-diagnostics of aging, based on the fact that today the SENS program (the achievement of negligible aging by engineering methods) most fully describes the approaches to increase human longevity.
Many have heard about the glycosylation of proteins, the end products of glycation (CNG, AGE) and about the harm they cause to the body. But, it should be noted that the addition of sugars to other molecules is not always a pathology. Glycosylation itself is a very common and important physiological process in living organisms. Thus, a significant part of all proteins synthesized in cells undergo enzymatic glycosylation, which is necessary for their normal functioning.
Mainly glycosylation involves two glycans (carbohydrate portion of the carbohydrate-organic molecule ligament): N-glycans (linked to the amide group of asparagine) and O-glycans (linked to the hydroxyl group of serine or threonine). Due to aging, we are more interested in N-glycans. It is described that with aging, the spectrum of sugar chains changes, which are attached to immune proteins upon N-glycosylation. And such a change plays a key role in the age-related increase in total inflammation in the body. Thus, the level of glycosylated IgG antibodies can predict a person’s biological age even more accurately than telomere length [1].
In addition, two more glycans, NGA2F and N2AF, proved to be promising biomarkers of aging. In the framework of the European ––––– –––––––––––––––––––––– program, the GlycoAgeTest test, which determines the biological age of a person, was developed by the MARK – AGE European Biomarker Research Program, completed in 2013. It is based on the ratio of the number of glycans NGA2F (increasing with age) and N2AF (the level of which decreases with age). Another potential biomarker of aging and age-related diseases (cardiovascular and diabetes), described in the framework of the MARK – AGE study, was the glycoprotein clusterin, which is involved in the stabilization of protein structures [2].
We now turn to the consideration of the pathological part of this phenomenon. In addition to the enzymatic glycosylation regulated by the body, there is a non-enzymatic form of this process, the so-called. the Maillard reaction, which results in the appearance in the body of a variety of glycation products. It should be noted that the process of non-enzymatic glycosylation is practically not regulated. Although there is a possibility of "restraining" glycosylation through transglycation, in which glutathione, polyamines, thiols, free amino acids, for example, taurine, lysine, are used as "consumption". And also through the inactivation of methylglyoxal by the glyoxalase system: glyoxalase I converts methylglyoxal and reduced glutathione into lactoylglutathione, which is further metabolized to D-lactate by the action of glyoxalase II.

The structure of methylglyoxal
Early (products of Amadori) and late (or final) products of glycation are distinguished from the products of glycation. Nonenzymatic glycosylation occurs in several stages. This process begins with the fact that glucose and other simple sugars combine with the amino group and start a chain of further reactions. In the beginning, during the reaction between the aldehyde group of sugars and the amino group, an unstable aldimine group (Schiff bases) is formed, which can turn into a number of other, more stable compounds, early glycosylation products, the so-called. Amadori products. One of the early products of glucose attachment to a protein is Ne – fructosyl – lysine, which, in turn, degrades, forms various end products of glycation (CNG). Hydroimidazolones, derivatives of arginine, are formed in the greatest amount as CNG, modified with glyoxal, methylglyoxal and 3 – deoxyglucosone (3 – DG). Another well-studied CNG is Nδ – carboxymethyl – lysine (CML) and Nδ – carboxyethyl – lysine (CEL), as well as glucosepan and pentosidine, which are characteristic of protein cross-links [3]. One of the most easily identified types of CNG is pentozidine, which accumulates, for example, in the tendons of large human muscles (that is, where the rate of collagen turnover is slowed) since 20 years and its concentration increases linearly with old age.
Until recently, it was believed that glucose is the main substance for the formation of CNG. But the exact establishment of different rates of intra-and extracellular formation of CNG showed that this is not the case. Such sugars as fructose, glucose-6-phosphate and glyceraldehyde-3-phosphate have a higher rate of intracellular formation of CNG. The negative effect of glyceraldehyde on the molecule of the main contractile and cytoskeletal protein actin, leading to the formation of pentosidine and bityrosine crosslinks and loss of actin functionality, has been described [4].

Stitching collagen molecules with glucosean.
As suggested today, non-enzymatic glycosylation and CNG are closely associated with a number of age-related diseases, such as diabetes, rheumatoid arthritis, atherosclerosis, Parkinson's and Alzheimer's disease, amyotrophic lateral sclerosis, cataracts and oncological diseases [5]. Proteins subjected to glycation in the Maillard reaction, become aphysiologically cross-linked, losing their properties. Especially a big problem for long-lived proteins (for example, skin collagen molecules, according to several studies, have a half-life of 15 years, and cartilage is more than 100 years), which constitute a significant part of all proteins in the body - about one-third. Formed additional cross-links between molecules violate the functions of these proteins, which leads to a loss of tissue elasticity and is often observed during aging and pathologies.

Illustration (a) shows a diagram of collagen fibril and the formation of glucoseepan, which covalently binds the side chains of lysine and arginine. (b) The chemical structure of glucosopan is shown, which crosslinks lysine (blue) with arginine (red). (c) A molecular model of glucose is shown.
The involvement of glycated fibroblast growth factor β-FGF in the formation of fibrosis has been described. Glycation of the arg-gly-asp protein of fibronectin leads to a violation of vascular restorative ability and progression of vascular pathologies. CNG accumulates in the lens and retina with age. Crystallins, the major structural proteins of the lens of the eye, are susceptible to glycation and cross-linking. Glycation of lens proteins leads to cataracts. Glycated hemoglobin, which is used to determine the average level of glycemia over the past 3 months, loses the ability to carry oxygen to tissues, which negatively affects many chemical processes.
It is also known that protein glycation and the formation of CNG is accompanied by an increase in free radical activity and lipid peroxidation, which contributes to the development of age-related diseases. This is due to the fact that the process of glycation of proteins triggers the auto-oxidation of sugars, accompanied by an increase in the production of reactive oxygen species (ROS). In addition, the binding of CNG to the CNG receptor (RAGE) also leads to the formation of reactive ROS and the subsequent activation of the oxidative stress-sensitive transcription factor NF-kB, associated with inflammation and atherogenesis.
In turn, activation of NF-κB increases RAGE expression, creating a positive feedback cycle that enhances the production of inflammatory promoters. In addition, the interaction of CNG-RAGE activates NADPH oxidase (a complex of enzymes that produces superoxide), which increases intracellular oxidative stress. And an increase in oxidative stress with NADPH oxidase in response to AGE-RAGE interaction activates, in turn, NF-κB. And here we can observe a whole series of “vicious circles” - cycles with positive feedback.
Glycation of the mitochondrial respiratory chain proteins also contributes to this process: a malfunction of the respiratory chain is a constant source of superoxide radicals. In addition, the formation in mitochondria and other structures under the influence of glycation of non-degradable CNG may be responsible for the implementation of such a phenomenon as "metabolic memory". When in patients with type 2 diabetes, strict glycemic control no longer prevents the development of complications associated with cardiovascular diseases. It is assumed that non-decomposable CNGs play a major role in this phenomenon [6].
Also during glycation, an increase in inflammatory processes occurs. So, during collagen glycosylation, accumulation in the extravascular matrix of the end products of this process, which the body perceives as foreign, occurs. Because of this, an immune response will occur, during which immune cells - macrophages - will increase the secretion of pro-inflammatory cytokines (TNF-α, interleukin-6, etc.), which are known for their ability to adversely affect insulin resistance and atherogenesis [7].
In addition, the “cross-linked” proteins change the structure (nanotopography) and increase the rigidity of the extracellular matrix and, through the mechanoreceptors of the membrane and the cytoskeleton of the cells, together with the signaling molecules, affect gene expression, the function of cells, tissues and the organism as a whole. Moreover, thanks to proline, collagen molecules conduct weak electromagnetic waves generated by cells and tissues, and, perhaps, in addition to mechanical and chemical signals, form a single bioelectric signal system of the body. It is interesting to note that by changing the topography of the medium or electromagnetic radiation, it is possible not only to control the cell cycle, but also to transform adult somatic cells into stem cells without the help of viruses with the vector of Yamanaka factors.
Even minor changes in the shear modulus and viscoelasticity of the extracellular environment have a strong effect on the cells — the “old” extracellular matrix can significantly limit the effectiveness of the use of senolysis and stem cell therapy. So, “young” fibroblasts rapidly grow old in the old matrix and vice versa - the “old” cells lose the signs of the secretory phenotype associated with aging in the “young” matrix.
What are the causes of glycated proteins and CNG? It is believed that the main one for modern man today is junk food. The roasted brown-black crust on the products represents the collection of CNG, which are formed due to the heat treatment of products (roasting meat, potatoes, etc.) at high temperatures. The record for the number of formed KKE is considered to be fried bacon. In addition, the consumption of foods containing a lot of fast carbohydrates (confectionery, sugary carbonated drinks, etc.) can also contribute to protein glycation and the formation of CNG. There are products that, on the contrary, help to resist the accumulation of CNG - black radish, radish, broccoli, due to the substance contained in them sulforaphane. As it is not surprising but along with the gourmets regularly supply themselves with higher doses of CNG and smokers. Tobacco smoke contains glycotoxins, which react with lysine and arginine residues to form CNG. This is probably why smokers have chronic inflammation of the respiratory organs [8].
As already mentioned, in addition to the influence of external factors, the formation of CNG in normal conditions also occurs endogenously (that is, inside the body) under physiological conditions. Although this process is slow and insignificant, it is significantly enhanced with an increased concentration of free radicals, insulin resistance, diabetes mellitus and an increase in blood glucose levels. Accumulating in the body, CNGs drag people into the “swamp” of pathological processes, from which it is not so easy to get out.
According to the figurative expression of SENS authors, protein crosslinks act as molecular “handcuffs” that bind protein molecules, disrupting their function. The solution to this problem, the authors of SENS see in the development of drugs that can react with cross-links and break them without destroying the other structural features of the molecules. In their opinion, a favorable circumstance in this process is that crosslinks, which occur as chemical accidents in the structures of our protein molecules, have a very unusual chemical structure, which normally does not occur in substances produced by the body. Which should facilitate the search and creation of therapeutic agents.
In addition, the authors of SENS define a number of promising approaches to solve this problem. Such as the search or development of enzymes, instead of drugs, to destroy crosslinks. As well as the development of "disposable" proteins that would destroy the cross-linking, and then they themselves would be destroyed in the process. It is known that such proteins exist for other purposes, for example, the DNA regenerating protein MGMT [9]. In favor of the approach to the search for enzymes, says the fact that living organisms described deglykiruyuschie enzymes. Thus, fungi and bacteria found anti-glycation enzymes amaadoria, fructozolysin-6-kinase frlD and fructosezolysin-6-phosphate-deglycase frlB, which act on low-molecular-weight amino acid compounds with sugars [10]. In vertebrates, the enzymes fructosamine-3-kinase (FN3K) and its related protein FN3K-RP are found.
It is believed that glukezepan has the greatest impact on the course of the diseases of the elderly person and therefore is a priority target for anti-aging therapy.
Unfortunately, glucoseepan was not targeted for previously developed anti-crosslinker drugs such as Alagebrium / ALT-711 (Wolffenbuttel et al., 1998), C36 (Cheng et al., 2007), TRC4149 (Pathal et al., 2008 ), and he only has to prove his role in the mechanisms of aging. These substances were aimed at neutralizing carboxymethyl lysine, the most common late product of the Maillard reaction, which accumulates in the body in diabetes.
Currently, a group of David Spiegel from Yale University is working on the synthesis of antibodies against glucoseepan-containing proteins. Aubrey de Gray confirms the importance of research on glukezepan and recently announced the registration of the company Revel, on the basis of which research initiated at the university may be continued.
However, it is likely that neither antibodies nor enzymes, due to their size, will be able to penetrate between collagen fibrils. Moreover, the broken cross-links of collagen are restored after the end of the drug intake, which will require a repeated course of therapy.
Therefore, artificial enzyme-like catalysts, the dimensions of which may be several times smaller than the sizes of the original enzymes, seem to be a more interesting alternative. Such molecules with a given catalytic activity — spioligomers (spiroligomeres) are being developed by the team of Christian Schaffmeister from Temple University.
As noted earlier, since the end products of glycation accumulate during aging, their quantitative determination can be an accurate and reliable biomarker of aging. However, both the detection of CNG in the body and the early diagnosis of changes in the composition and structure of the extracellular matrix are difficult due to the nature of the changes limited by organs and systems and the need for a multiple biopsy. Even in one organism, the results are very different depending on the place of biopsy and the type of glycation end products studied.
Available methods for determining the end products of glycation in the skin, such as autofluorescence, that is, without the use of special dyes (the “AGE-READER” device manufactured by the Dutch company DiagnOptics BV) do not provide accurate data and many factors can affect the measurement results - salt balance of the body.
The main and most accurate method for quantifying CNG in the body today is the method of chromatography combined with mass spectrometric detection. Mass spectrometry makes it possible to identify proteins with a high degree of reliability and to determine their amounts in complex protein mixtures.
An enzyme immunoassay is also used to determine the level of CNG. But this method has a number of qualitative limitations (insufficient specificity of antibodies, the effect of free glycation products, etc.). Therefore, it is considered that it is advisable to carry out it in combination with mass spectrometry. CNG can also be identified by the total intensity of their fluorescence. What also has its limitations (most of the CNG do not quantitatively fluoresce, therefore, cannot be determined) and allows you to more accurately determine the low molecular weight peptides of CNG and free glycation products.
One of the promising biomarkers, showing not only the accumulation of CNG, but also an increase in the risk of all-cause mortality, is plasma carboxymethyl-lysine (CML). KML is one of the dominant CNG in the body, both circulating and tissue. In addition, KML is the only CNG that acts as a ligand for CNG receptors (RAGEs). The binding of RAGE to KML leads to an increase in the generation of free radicals, activation of the pathway of the nuclear factor Nf-κB and an increase in the level of inflammatory mediators (such as tumor necrosis factor alpha, interleukin-6 and C-reactive protein). It is known that KML accumulates in the large blood vessels with age. And high concentrations of this CNG in serum are associated with greater arterial stiffness, a powerful risk factor for the development of cardiovascular pathologies and mortality from them,
It is also known that elderly people with cerebrovascular diseases, elevated levels of KML are found in cortical neurons and brain vessels, which is associated with the severity of cognitive impairment. As part of the 6-year study of Invecchiare in Chianti, which was attended by 1,013 people over 65, it was shown that the average plasma concentration of KML, which was measured by ELISA, was significantly higher in those who died of all causes than survivors [13]. In 2018, a group of Danish and Swedish scientists described a new monoclonal antibody, D1-B2, aimed at KML, which has good potential in ELISA to detect this CNG. [14]
Previously, using coupled liquid chromatography along with mass spectrometry, a number of CNG (glucosean, DOGDIC, MODIC and GODIC) were identified that accumulate in tissues during aging and pathology and can be used as markers for pathophysiological processes [15]. The method of high-performance liquid chromatography allows to detect two more classes of CNG associated with aging and diabetes - GOLD and MOLD [16].
Review authors: Denis Odinokov, Alexey Rzheshevsky.
Bibliography:
- Krištić J, Vučković F, Menni C, Klarić L, Keser T, Beceheli I, Pučić-Baković M. et al. Glycans are a biological and biological ages. Gerontol A Biol Sci Med Sci. 2014 Jul; 69 (7): 779-89.
- Bürkle A, Moreno-Villanueva M, Bernhard J, Blasco M, Zondag G, Hoeijmakers JH6, Toussaint O, Grubeck-Loebenstein B, Mocchegiani E, Collino S, Gonos ES, Sikora E. et al. MARK-AGE biomarkers of ageing. Mech Ageing Dev. 2015 Nov;151:2-12.
- Ahmed N., Thornalley P.J. Роль конечных продуктов гликирования в патогенезе осложнений сахарного диабета. Российский медицинский журнал. 2009. №9, стр. 642-51.
- Федорова М. А., Благовещенский И. Ю., Филимонов В. Б., Кулева Н. В. Неэнзиматическая модификация актина in vitro под влиянием факторов окислительного, гликоокислительного и нитрозактивного стрессов. Вестник СПбГУ. 2006, №2, с. 51-59.
- Ансари Н.А., Рашид З. Неферментативное гликирование белков: от диабета до рака. Биомедицинская химия, 2010, том: 56(2), 168-178.
- Герасименко О.А. Конечные продукты избыточного гликозилирования как потенциальная мишень «выключения» метаболической памяти. Эффективная фармакотерапия. Эндокринология. 2011. № 4.
- Bernheim J, Rashid G, Gavrieli R, Korzets Z, Wolach B. In vitro effect of advanced glycation end-products on human polymorphonuclear superoxide production. Eur J Clin Invest. 2001. Dec;31(12):1064-9.
- Mullick AE, McDonald JM, Melkonian G, Talbot P, Pinkerton KE, Rutledge JC. Reactive carbonyls from tobacco smoke increase arterial endothelial layer injury. Am J Physiol Heart Circ Physiol. 2002 Aug;283(2):H591-7.
- GlycoSENS: Breaking extracellular crosslinks.
- Monnier VM, Sell DR. Prevention and repair of protein damage by the Maillard reactionin vivo. Rejuvenation Res. 2006;9(2):264–273.
- Szwergold BS1, Bunker RD, Loomes KM. The physiological substrates of fructosamine-3-kinase-related-protein (FN3KRP) are intermediates of nonenzymatic reactions between biological amines and ketose sugars (fructation products). Med Hypotheses. 2011 Nov;77(5):739-44.
- Semba RD, Najjar SS, Sun K, et al. Serum carboxymethyl-lysine, an advanced glycation end product, is associated with increased aortic pulse wave velocity in adults. Am J Hypertens. 2009;22:74–79
- Semba, R. D., Bandinelli, S., Sun, K., Guralnik, J. M., & Ferrucci, L. (2009). Plasma Carboxymethyl-Lysine, an Advanced Glycation End Product, and All-Cause and Cardiovascular Disease Mortality in Older Community-Dwelling Adults. Journal of the American Geriatrics Society, 57(10), 1874–1880.
- Wendel U, Persson N, Risinger C, Bengtsson E, Nodin B, Danielsson L, Welinder C, Nordin Fredrikson G, Jansson B, Blixt O. A novel monoclonal antibody targeting carboxymethyllysine, an advanced glycation end product in atherosclerosis and pancreatic cancer. PLoS One. 2018 Feb 8;13(2):e0191872.
- Biemel KM, Fried DA, Lederer MO. Identification and quantification of major maillard cross-links in human serum albumin and lens protein. Evidence for glucosepane as the dominant compound. J Biol Chem. 2002 Jul 12;277(28):24907-15. Epub 2002 Apr 26.
- Chellan P, Nagaraj RH. Protein crosslinking by the Maillard reaction: dicarbonyl-derived imidazolium crosslinks in aging and diabetes. Arch Biochem Biophys. 1999 Aug 1;368(1):98-104.
