Registration of medical devices: how many mice will suffer in the process
One may say that there is something wrong with medicine in Russia, but we have one of the best consumer protection in the world. Compared with our European standards for medical devices - this is just a paper certificate.
We have in recent years need to prove any effect. That is, if you meet the ointment from wrinkles, and it does not remove wrinkles, you can complain to the Federal Service for Supervision of Consumer Rights Protection. And somewhere far away at the production one can hear the sound of vaseline opened by a can. If you pophlohelo after you took any therapeutic balm, you can also complain. And again - chpok, someone will come with a check.
This whole story of toughening the requirements touched us seriously a couple of years ago when we registered Blefarogel. But let me just tell you in detail how much Vaseline, tears and snot we spent on this process.
4 circles of hell
The basis for registering medical devices is Resolution No. 1416 .
It describes the rules and procedures for registration. In order to undergo this rather complicated procedure, it is necessary to carry out the following tests:
- Toxicological (proving that the product is safe in terms of materials and substances used in the formula).
- Technical (proving that the product in its properties and technical characteristics meets the requirements of specifications and operational documentation of the manufacturer, as well as the fact that the production process will be uniform and will not need to check every single element of the party).
- Clinical (proving the effectiveness and safety of solving a medical problem).
- And the examination of quality, efficiency and safety, where the commission collects all the data on the product in one pile and decides on the possibility of its registration and release to the market.
Important point: by law, a medical device cannot be treated. That is, if it has a healing effect, then this medicine is a completely different category. Medicament can be something auxiliary in the process. For example, if a tonometer is recorded, it is necessary to prove that it measures pressure, that the sleeve at the points of contact with skin will not cause allergies or various dermatitis, that pressure will be measured with a prescribed tolerance throughout the life of the device. In the case of a duck, it is necessary to prove its hypoallergenic and safety for the patient. In the case of our gels, the main thing is to undergo Roszdravnadzor, including toxicology.
It used to be relatively simple with toxicology. Today we must begin with the analytics of components and their interaction. What is important concerns not only the product itself, but also the packaging. Previously, only the gel would be checked, and now, according to the new standards, the gel and packaging (bottle with cap) of all the used packaging and colors, as well as their interaction. This is done to ensure that the vial substances do not change the properties of the gel, migrating into its composition.
First you need to carry out litobzor, that is, to find information in the form of articles and reviews on registered counterparts, proving their effectiveness and safety, find a practice on the active substances and prove that they are safe in the mixture.
Why the first stage of litobzor? Let me remind you that we do not have medicine, but medical equipment. There is no need to test a new formula. More often.
It looks like this: we refer to articles for examination, that is, we find journals in archives, notarize screenshots and copies so that the commission itself would not search later.
Then we need feedback from clinical practice on registered analogues. That is, we must find publications (or take the once-registered closest analogue and test how it solves this problem). Based on the information collected on registered analogues, an act of evaluation of the results of clinical trials of medical devices is drawn up.
Next, with a package of documents confirming the conduct of all the steps mentioned above, as well as with other additional documents for a medical product, we are going to Roszdravnadzor for registration.
How is the process going
First, an application and a dossier for the future product are submitted to Roszdravnadzor. The statement describes the purpose, risk class, scope and other information about the medical device, as well as information about the manufacturer. In addition to the application, a set of documents is attached: protocols, acts of all tests carried out, technical documentation (TU), operational documentation (instructions for use, labels), photographic images, risk analysis report, information on regulatory documents, certificate of qualification tests, as well as lease agreements and other manufacturer documents.
That is, at the time of filing an application, all internal tests must be completed (in our production in our own laboratory they are made harder than in state laboratories, because it is elementary more profitable to go in the first time and not to face batch responses later in the sales process). Then donate toxicology to one of the laboratories with state accreditation.
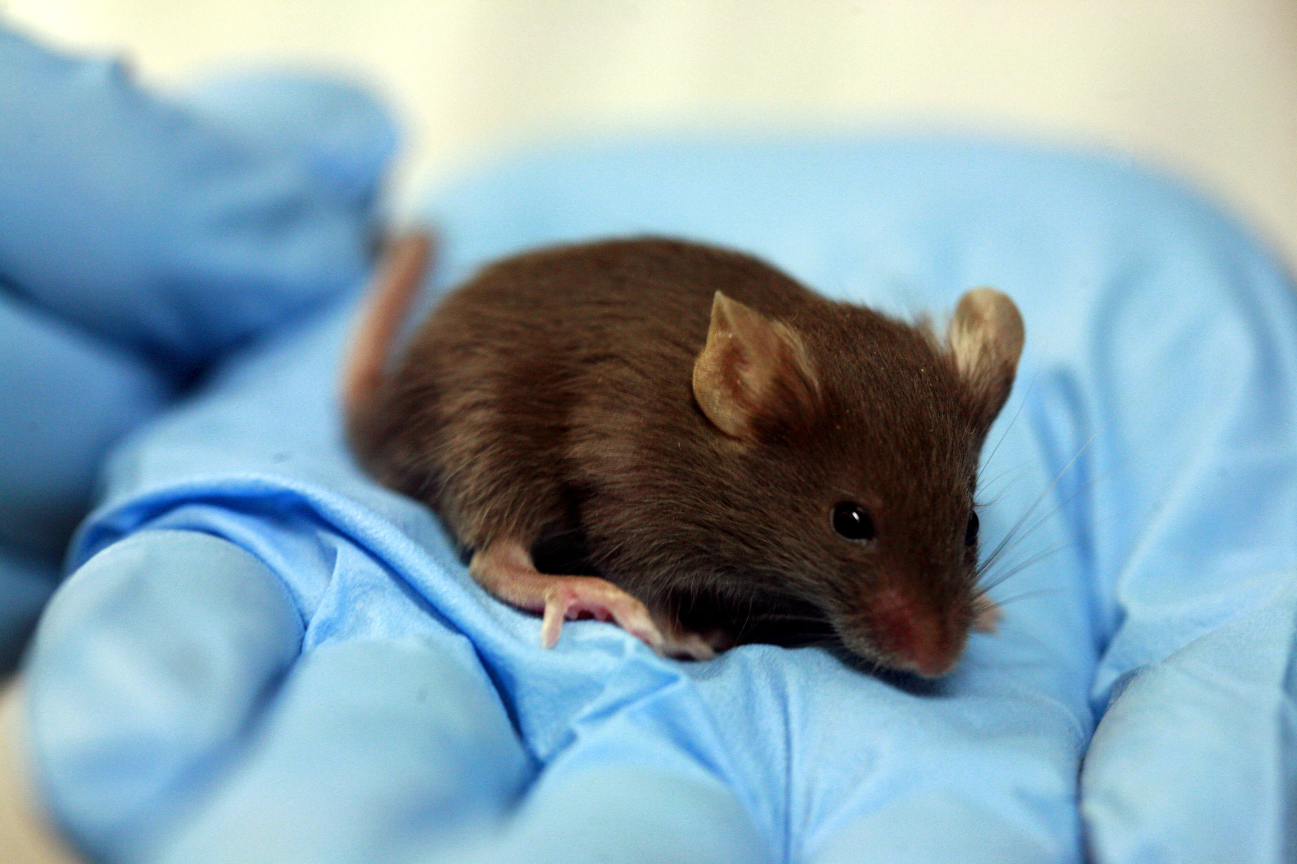
In our case, for example, Blefarogel (eyelid hygiene for the prevention of dry eye syndrome) - this is a gel that is applied to the eyelids and face skin. Toxicology was a mouse. I don’t know what exactly was done with them, but I suppose that two areas were shaved on each animal, one was smeared with gel, the other was left or smeared with something neutral. And watched the mouse. Inside the mouse, fortunately, did not shove. (Well, almost did not shove - the fact is that in the safety data sheet [or MSDS - material safety data sheet] of propylene glycol there is data on the lethal dose when taken orally. It should be 4 grams per mouse [it physically breaks at the same time, most likely] And somehow they set this dose ...) But back to the more successful mouse companions, which are simply smeared. They have neighbors, who got clean bottles from the product, in which they pour water and defend. Then they are smeared with this water from the vials to check that plastic over time does not release something dangerous. Or not: the exact method is not disclosed to manufacturers, there is a high probability that this water from the vial is simply chemically analyzed and already under suspicion is entered intradermally.
We feel sorry for the mice, but the approach is this: if it were not for our tests, they would not have been born at all. In general, they are not tortured there (at least, we are already entering with a ready study on ourselves). We also know that laboratory assistants have warm feelings for mice, comparable to a chicken in a village dweller's love. And we suspect that at the end of the research the mouse is not completely utilized, but is sent for rehabilitation before new tests. But this is only a theory: if there is someone here who can tell about their fate, it would be great.
This application must be accompanied by technical studies. Specifically, write a TU (normative document for a medical product that establishes technical requirements) and prove that the medical device complies with it. Experts agree on our terms. Then the stability of serial samples and the compliance of their “dossier” of the product are checked there: color, smell, electrical conductivity, attenuation of the ultrasonic signal, and so on - all that is indicated. At this stage, it is often necessary to reformulate one or another word in the product description. Our Mediagel has different viscosities: this is one registration certificate, but three sets of tests - for medium viscosity, a thinner and denser gel.
We carry out the same regular checks for compliance with specifications at our own production. Only once in the entire practice was there a case when we approached the permissible test limits, we usually go exactly in the center of the tolerances between the borders.
The risk class of a product is determined by its purpose, method of use, duration of use, invasiveness and other criteria. We have ultrasound gels, for example, the duration of skin contact for up to one hour is first class. And in the second class, for example, get gynecological mirrors, different things for newborns.
There is a difference in clinical trials. The first class - clinical trials can be done immediately. Second class and above - the clinic only after the official permission of Roszdravnadzor. Prior to clinical trials, the development team has the right to test only on itself. The goal - to make sure the main effect. The goal of the beta is to understand the possible side effects and various interesting cases like "against the background of taking other medications." Clinical trials begin when the safety of a medical device is proven in theory.
I note that if it is not about medical equipment, but about cosmetics, then research is conducted on volunteers in clinics accredited for scientific research of this type.
In practice this way: the commission finishes the analysis of documents (with the exception of medical devices of the 1st risk class of application and medical devices for in vitro diagnostics), then they send a letter and give 50 working days to conduct clinical studies and provide them with the results. For most of our gels, all clinical practice was obtained before the requirements were tightened, so there were no difficulties. But now we have a target order from ophthalmologists for the new Blefarogel Forte - it’s like Blefarogel-2 with sulfur compounds for demodex, only for neglected cases. If Blefarogel-1 is “so that the eyes do not hurt while I am sitting at the computer,” Blefarogel-2, “so that there is no inflammation from the tick and he has nothing to eat on his eyelids,” then Blefarogel Forte will be “genocide of ticks at any cost”. In the sense that we deliberately increase the risk of allergies, increasing the proportion of active ingredients, but recombining the formula so that it helps in particularly difficult cases. Since this is done at the request of doctors who only foresee that they need such a tool.
The end of the epic
Further, the completeness of documents in Roszdravnadzor is checked: an implementing consultant is allocated who simply checks the package. Documents are scanned and sent to an expert organization in the department of Roszdravnadzor. As a result, the Roszdrave documented a report based on the totality of all documents. Makes it an expert group. If everything is good and the documents correspond to each other, then they give permission for state registration.
From this moment nothing can be touched at all. Replace the letter on the label or replace the icon automatically means that you need to re-register. We were asked to somehow change the label Mediagel-s to Russian-English. This means re-registration. It took half a year to add an English text.
As an expert process, in many respects it is associated with concrete interpretations of rather complex norms. This means that several times you can submit documents using the same scheme, and then it turns out that you will need a new piece of paper. In general, this is a kind of coincidence: they can wrap up at each re-submission. At the same time, the old state registration remains and does not disappear anywhere, but we lose money for tests and pay a large state duty even for registration and introduction of changes. And spend the state mice.
Next you have to wait for the order on paper. When there is an order, the product will go to the registry, that is, to the site where the pharmacies look for what is what. Only from this point can something be done. The pharmacy does not take products that are not in the Ministry of Health’s Office if it is not cosmetics, of course.
Only at this moment you can begin production. Without a Ministry of Health number, we can not only sell, but even produce in Russia.
Everything, from this point on, we do medical equipment serially. From experience: it is very important to have a developed quality control laboratory, because any complaint means a thorough inspection at work (the commission, judging by the behavior, cannot be returned without reporting any violations) and stopping shipments. In this regard, we prefer to carry out acceptance and periodic tests independently and overlap studies on small samples with our own. I mean, 10 volunteers from the standard is not enough, but 100 volunteers are already a more or less representative sample. In the long term, larger-scale research always pays off, and for me it is a big mystery why other manufacturers do not always do that.
In the next post I will try to tell you about another important stage of research - the challenge-test: this is when samples are specifically contaminated by pathogenic bacteria. In no case should this part of the research be done somewhere close to production or research laboratories, so the challenge tests are taken out.
