DIY dishwasher powder: how not to dissolve the dishes and not to repeat my mistakes. Year of experiments

Previous publications:
→ Powder brain or how to make powder for the dishwasher to 9.7 times cheaper
→ DIY powder for the dishwasher: dismantle industrial facilities and improve the recipe
has been over a year since the last publication of a series of domestic alchemy dedicated to
Madness and common sense

Planning problems: If you suddenly come up with a brilliant idea to soak a roll of toilet paper with calcium nitrate and dry it in the oven - chase that thought. Bad idea.
For those who have just joined this orgy, let me remind you about this. Once I became very bored and I decided to dig into the chemical composition of dishwashing detergents in PMM. As a result, I came to the conclusion that most often the cost of such funds is highly overvalued and the composition is often non-optimal. Strangely enough, but I finally saw a bunch of reposts of those publications and a considerable number of people who also decided to experiment. The only thing I wanted to warn you to be sensible. If you do not understand what exactly you are doing - do not do it. If you are reluctant to read the composition and understand it - do not even try. Common sense has not been canceled.
What do I use now?
As I already wrote, one of the key pieces of composition analysis is the possibility of optimizing efficiency, and not just cost. I was repeatedly rightly poked my nose at cheap factory options. Strangely enough, but I finally settled on the powder from the hypermarket Tape under their brand. Mediocre, but happy. All alchemy turned into a slightly different plane - to facilitate the washing of particularly cruel contaminations, taking into account their specificity. Well, in the direction of non-standard applications of the dishwasher, as I also tell.
The current algorithm is as follows: if the pollution is moderate, we use the standard cheap powder. If this is a breast fish with fat dropping from a burnt pan, use heavy options. Moreover, in the light version, we simply use alkaline mixtures with detergents at the preliminary washing stage, and in especially heavy ones we use very cruel things like alkali alkaline with complexing agents.
Basic component requirements
We have several requirements for the substances we use:
- We should not be poisoning them, including in the long run.
- Substances should not harm containers
- Substances should not damage the dishwasher
- They must fulfill their function.
- The house should stay in place, along with sewers and residents
- State Drug Control and radioactive materials control services should not be interested in experiments
Toxicity
The easiest way is to use those things that are certified for contact with a person. Even if it is a washing powder, it still passes the tests for washability, toxicity and allergenicity. This is the main source of simple extraction of detergents. It’s just not so easy to find a surfactant in the pure state, and planing soap in MMP is a hopeless and fraught party with a foam party. Fluorescent dye from powders that contain it, apparently does not remain on the dish. This is checked by tbl :
I just checked a plastic plate and a metal pan from a source of hard UV radiation: I did not notice re-radiation in the visible spectrum (I myself currently use eared nanny, because I did not find a biolan without an optical dye).
However, I would recommend searching without the type of Biolan. Still, the washability depends on the location of the dishes and something can remain.

In the same FixPrice there is a powder with non-normalized foaming of Zif Ekonom (thanks to heathen ) The
second variant of simple and non-toxic components is easily soluble simple salts. I do not mean, of course, any kindness like sodium cyanide or soluble mercury salts. Although even in this case, probably nothing will happen. The main feature of such components is good solubility and, accordingly, complete washability. First of all, these components perform three main functions:
- Binding of hardness salts or transfer them into a soluble form (sodium carbonate, EDTA, tripolyphosphates)
- Alkalization of the medium for the alkaline hydrolysis of organic matter (sodium hydroxide, sodium carbonate)
- Oxidation of pigments from tea, coffee and other things (percarbonates + TAED)
Safety for dishes and dishwasher
Basic things that may suffer:
- Gentle enamel or covering dishes - it is generally better to wash your hands
- Aluminum things, elements and their alloys like silumin - personally killed the meat grinder parts
- Expensive glass (sorry) is alkaline and gradually grow cloudy
- Low-grade stainless steel - can corrode when solutions are highly aggressive
- Stainless steel is more resistant like the walls of the PMM itself - it is very difficult to damage
- Plastics are not resistant to alkalis - can degrade. These materials are usually not used in PMM materials.
- Rubber seals in PMM highways - are afraid of organic solvents and some organic acids like acetic acid

I did not find my photo, I found a similar one.
Most often aluminum falls under the distribution. The reason is that it is always normally covered with the thinnest molecular film of aluminum oxide, which protects it from further oxidation. But in an alkaline environment, especially in strongly alkaline, it dissolves, and aluminum begins to react with water. All this ugliness is accompanied by heating and release of a large amount of hydrogen. Well, you understand.I used it when I bore condoms out of boredom with hydrogen, putting them on glass bottles with NaOH solution (Mole for cleaning pipes) and aluminum foil. I realized my mistake when this horror quickly warmed to 100 degrees and began to boil. And throw is not an option. As a result, at the same time it was necessary to cool the bubbling filth with alkali vapors with cold water. There was a lot of hydrogen, everything flew right) But I don’t want to repeat. At the household level in PMM, it threatens blackening and wild metal corrosion. In particular, beautiful anodizing is washed off and everything is fucked with shells.
Please use goggles and signets if you decide to work with alkali so! This is really very dangerous.
What to do if your new skillet or saucepan, for example, has a bezel at the bottom, similar to aluminum? I adhere to the principle of “Get washed or die!” . Dishes that do not stand up to the dishwasher are dead dishes. The weak in spirit can look for the manufacturer's recommendations on this.
Corrosion of glass is also a problem. Oddly enough, but the glass is slightly soluble in water. In aggressive alkaline solutions, individual ions are washed out of the material, which leads to rainbow stains inside the glasses and glasses themselves. In soft water, this process goes faster. In my opinion, here we must treat the dishes as consumables, and wash hands with especially valuable services.
Corrosion of metals is not so acute. Most alloys easily tolerate even very aggressive solutions. Gentle steels corrode beautifully and in ordinary hot water. Moreover, even regular powders and tablets very cheerfully cause similar effects. The stainless steel of the dishwasher chamber itself is almost unkillable. Even aggressive chlorine plus alkaline medium left no trace. Apparently, the material used is similar to washing machines. If you have a lot of metal utensils and rust spots are troubling you - do not use too many percarbonates. Hissing hydrogen peroxide in hot alkaline water is a very aggressive environment. Especially refrain from chlorine oxidants - I generally recommend using them carefully and in special cases, I will tell below.
Plastics and rubber seals to alkalis resistant. These are usually high molecular silicones. Still, PMM is designed for alkaline sink by design. However, it is believed that there are a number of substances that can damage various compounds in the device. Acetic acid causes some types of plastics and rubbers to soften. It is also unacceptable to use any organic solvents such as acetone and others. The exception is very low concentrations of ethyl and isopropyl alcohol in rinses.
Gentleman's set of powders
Oxidizing agents

Oxidants are needed for two tasks. Oxidize something colored or nail something smelling. With both tasks they cope well. Basically, the simplest oxygen oxidizer is sodium percarbonate + TAED (activator). The easiest way to take them in FixPrice is as much as 50 rubles per 400g. Greens are the most evil. They also contain hydroponic (hydrogen peroxide in urea) and Trilon-B (complexing agent). Better to take them.
For very hard things, you can use sodium hypochlorite, which is “Whiteness”. Splits into eerie chlorine and ordinary table salt. Chlorine reacts safely and everything oxidizes, the excess evaporate. May cause corrosion of morally unstable steels. I have successfully applied to sterilizing virus-infected flower pots. I had a plantation Trinidad Moruga Scorpio ( veryhot peppers). But then some unknown plant plague ruined everything. A cool pots of plastic. In short, I stuffed everything in the dishwasher and washed everything from the heart with chlorine. Sterile and perfectly clean at the exit.
Detergents
A key feature of native surfactants for dishwasher - low level. However, as experiments have shown, even non-normalized foaming powders and liquids can be used. Another thing is that they should be dispensed very carefully . Foam - sadness for the pump, which is designed for incompressible fluid. As a result, it can overheat, if absolutely everything gets foamed. The second problem - foam can crawl out and flood everything. At KPDV at the top of the post is just the highest possible level of foaming. Therefore, any washing powders, especially not for automatic machines, must be very carefully dosed. I use a very hardcore version - I use Fairy. But the amount of not more than 5 ml. Very good for pre-washing with washing soda (sodium carbonate). Be careful with him! Foaming brutally!
Alkalis
The easiest option - washing soda. Just do not fry the usual in the oven, as I suggested in the first post. Just buy ready. This is sodium carbonate, worth a penny. It gives a fairly alkaline environment, washes in combination with surfactants very well.

A mixture of NaOH and Fairy. Washing the tank after a greasy duck.
Disclaimer
Alkalis are wildly dangerous! Please, be careful! You can suffer greatly. Aggressive deep burns
The toughest option that eats even the meat scale from broths is sodium hydroxide. NaOH - hard alkali in a concentrated form. Recently, for the experiment, I spread a couple of rat legs at work there. For a couple of hours, everything went into solution. But it is a concentrate. We will follow the standard industrial detergents, which imply a 0.5% alkali solution. This is a proven and safe concentration. At least regular solutions work like that. This is approximately 20 grams of powder per 4 liters of water, which corresponds to the usual PMM bay. Where to buy it? The easiest option - dry powders for cleaning pipes, they are also “Mole”. Just need to carefully watch the composition. I took a very good version of Ribbon - Chirton red powder :

In the composition of> 30% NaOH, 15-30% filler, 5-15% of the complexing agent (EDTA, apparently), <5% functional additive (xs that, but probably anti-caking or something like that).
Very important!
Do not try to take a green bag. There is a composition of alkali and aluminum powder. Remember how it reacts? You clearly do not need boiling liquid with aluminum and hydrogen.
More kosher option to buy in the same Pro bank of pure hydroxide and not suffer. In general, the alkali helps to wash the very brutal pollution. Can damage especially delicate dishes, be careful.
Complexing agents
Trilon-B, he's EDTA and his ilk. Awesome thing. Allow to convert salts and metal oxides into soluble form. At the same time, pure metal does not touch at all. I have copper inserts in the bottom of the pan, so they begin to shine with bright pink copper when I take it out of the dishwasher. In theory, if you are in a large city, you can freely buy a few kilograms of Trilon-B for a penny. His bags are used in boilers and other places. It is safe - it is in principle an antidote for poisoning with metal salts.
Trilon-B in combination with organic acids can be perfectly used to eliminate corrosion from tools and other pieces of iron. Rust gently washed off. This is the question of washing rusty nails) If you need to wash specific metal ions, then you should take into account the pH table in which it is active for different metals. For iron, these are acidic solutions. For example, citric acid is well suited.
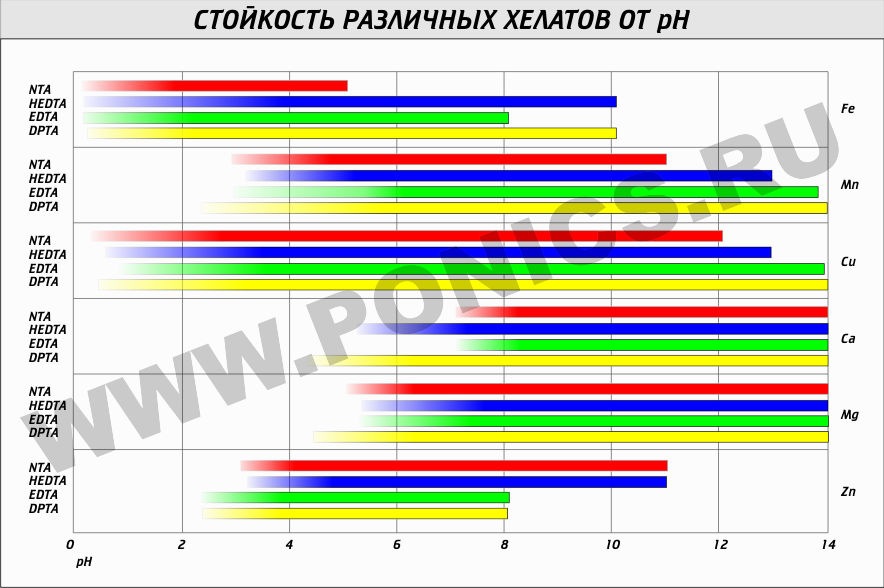
Other strange applications of the MMP
A dishwasher is, in fact, a rinsing unit with hot, aggressive solutions for anything. Therefore, the range of application is very wide:
- Wash not particularly dirty potatoes. For cooking in a uniform. Short programs without powder.
- Washing greens. Short programs in cold water without powder.
- Wash lightly rusted tools. Citric acid, Trilon-B
- Washing and sterilization of plastics, glass and other things. Alkalis, oxidizing agents (chloric, if absolutely necessary)
- Washing glasses for the oven from fat. Detergents, alkalis (NaOH)
- Any rubbish to your taste within reasonable limits.
Acknowledgments
Many thanks to everyone who tested all this disgrace with me. Sorry if I didn’t mention someone: BubaVV , tbl , Urvin , unwrecker , sergku1213 , darthslider , Zzzuhell , heathen and everyone else. I will be glad to feedback in this thread. Moreover, the time has already passed and accumulated experience.
UPD
viavel unsubscribed as a manufacturer of powder and tablets.
The composition should be hydrotropic non-ionic pav approximately 1%. The list goes on: tff na 15%, trilon b 5%, salt oedfk 3-5%, sodium silicate 7-10%, sodium percarbonate 15%, activator taed 1.5%, enzyme protease 1%, lipase 0.5%, soda calcinated 25 -35%. Sodium sulfate or sodium chloride to 100%. This is a simple composition. The tablets are more complicated.
Purely subjective, the composition is very similar. It is going from the components of the Fix Price. Only enzymes problem.
UPD 2

Thanks iig . He showed a relatively inexpensive source of polyphosphate for one-time tests. Google on request " polyphosphate backfill ". It is used for filling polyphosphate filters with granules. Sodium polyphosphate (aka hexametaphosphate) is more expensive than tripolyphosphate, but much more effective as a surfactant and complexing agent. More active groups. There is a proposal to test it for those who are faced with those who are faced with the problem of plaque on dishes from hardness salts. You do not need to grind; just drop a few crystals into the container along with the powder. Must dissolve.
Of the common stores - Leroy Merlin: a small package and a large bank about a pound . The question with Trilon B is still open.
For tests, I want to call Markscheider , tbl , Urvin , unwrecker , darthslider , Zzzuhell , heathen .
UPD 3

Suddenly found even ion exchange resins for food water. GrisGreyler , as a last resort , you can replace the damaged resin for the dishwasher completely.
