Lithium-ion and lithium-polymer batteries: marketing tricks and common mistakes
I repeatedly come across articles and comments (in articles, however, much less often) using incorrect data or names, which are subsequently presented as arguments, although in fact they are erroneous from the very beginning. And these errors spread across all resources, including Hiktatimes.
With this article I would like to clarify some points and conduct a kind of educational program.
Right from the main point - there is no free lithium-polymer batteries in the technical sense of the word in the market. In the English-speaking world, this has already been sorted out, but in the post-Soviet space there are some costs in the terminology used by marketers. A small digression is not that they are not used in other regions, but at least there is an opportunity to check this information in their native language.
Any lithium-ion battery has 4 main components - two electrodes (anode and cathode), an electrolyte and a separator. All 4 elements have developed and are developing further. At the beginning of the research (1970s), two options were proposed for electrolyte — liquid or solid electrolyte. At that time, the solid electrolyte promised more prospects for operation - the electrolyte does not leak out if the casing is damaged, the element itself is more durable. The main drawback was and remains the high resistance of solid electrolyte, it negates the physical characteristics.
In fact, a decrease in the amount of resources allocated by companies to develop solid electrolytes occurred in the early 1990s, when Sony introduced a liquid electrolyte battery to the market. Sony itself in 1988 was confident in the future success of solid electrolyte.
Despite the orientation of the liquid electrolyte, companies have not stopped looking for alternatives. One of the options was the so-called hybrid electrolytes. In fact, they use a separator with small holes and the same liquid electrolyte. Although it feels dry to the touch, in fact the amount of electrolyte in it does not differ from that in a conventional battery. Both in principle and construction:
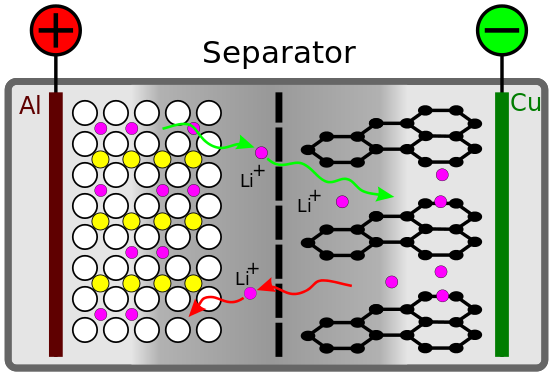
Schematic model of a lithium-ion battery with a LiCoO 2 cathode and graphite anode from Wikipedia in German.
These batteries are quite common, their commercial distribution began in the early 2000s, but physically and chemically they are the same lithium-ion batteries with liquid electrolyte and in general there are not very many of them.
One way to classify batteries is its case. Today there are three popular packaging methods:
The first type of battery is known for its use in laptops and Tesla cars (it uses the most common size of 18,650).
The second type is a modified cylindrical shape. Aluminum body, rectangle or square in cross section. Popular for stationary use and in transport.
The third type has a soft body and is not always equipped with a built-in protection system. In fact, a cheaper version of the prismatic cell. This type of battery is used in particular in mobile phones.
The last ones in the list are those “polymer” ones. They are so called for several reasons. The most arrogant way to marketers - the case of polymers, and therefore "polymer".
The second option is the use of a fine polymer separator. In fact, no different from a conventional lithium-ion battery.
The third option, which I have not met, is to give the name “polymer” based on the use of polymer elements as bases of cathodes, anodes, and other elements. Usually falls into a lot of batteries in a plastic case.
When developing the concept, the idea was that the term “liquid electrolyte” was understood to mean a liquid or gel-like solution of lithium salt, while the term “solid electrolyte” (solid electrolyte) was a solid state of matter. Since there was a desire to sell what was promised but what is not, today, even among researchers, gel electrolyte is added to the list of "solid" electrolytes, although its characteristics are still more hybrid. Therefore, it is possible to meet the description in scientific papers “solid gel electrolyte”, which some scientists consider to be misleading.
Developments are underway and in the future, the appearance of batteries with a real polymer electrolyte is possible. However, as of 2015, laboratory samples of polymer electrolytes based on organic chemistry did not show any tangible progress, because at the date of publication of the article in the foreseeable future there will be no mass withdrawal from liquid electrolyte.
There are several different types of lithium-ion batteries on the market. They have various names that allow you to describe their characteristics in terms of capacity or safety. In general, the following types can be found:
Immediately, you can notice the unevenness of titles. Some are named after the cathode, some after the anode. And if in the first case, you can still try to guess with a high degree of probability that the anode will be graphite, then in the case of the name by the anode, it remains only to guess. Also today, developments are underway and, in principle, a battery with a LiFePO 4 cathode and Li 4 Ti 5 O 12 anode can be found on the market , i.e. lithium-iron-phosphate lithium-titanate, which in this system do not have a simple marketing name. By reference - a scientific article of 2013 with tests of such a battery .
The reason for the existence of such a large number of cathodes and anodes of batteries in different battery requirements. Somewhere need more security, and somewhere capacity or power. You can get an idea of the stored energy based on the fact that each type of cathode and anode has a different potential, as can be seen from the images below (the potential of metallic lithium is chosen as 0 V potential, the voltage difference is greater - more power, energy density depends on the number of atoms lithium):

General scheme with potentials from the University of Kiel. Source
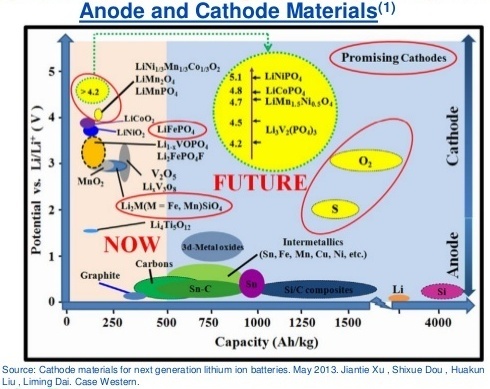
Material from a 2013 article by Jiantie Xu, Shixue Dou, and others. Source
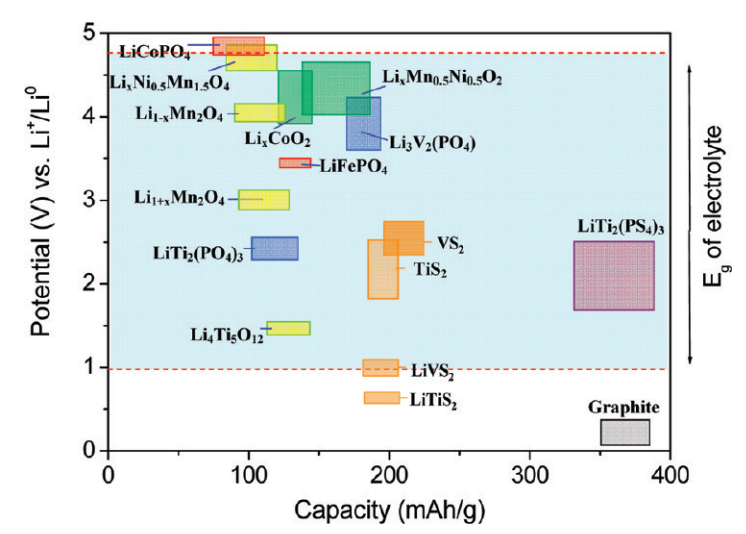
Another picture from the Purdue School of Engineering and Technology. A source
The general understanding of the causes can produce the following rough image communication potential elements and the possibility of metallization of lithium at very low discharge or thermal instability overcharge:

Pictures taken from lectures
most unsafe to operate on the market - the lithium-cobalt with a graphite anode, the safest - with the cathode LiFePO 4 and the anode Li 4 Ti 5 O 12 . Naturally, the presence of the BMS (Battery Management System) reduces the risks, but you should not neglect them, this system will not be able to prevent the same too strong discharge, which is critical for batteries with a graphite anode.
The most common and most common mistake is the opposition to the "ordinary lithium-ion battery." As can be seen above, there is simply no such thing as “ordinary”. And the difference in voltages can be very different for seemingly identical cathodes and the same for different sets of cathodes and anodes.
The second error, not so significant, related to the previous item, writing the material of the cathode LiFePO 4 as follows - LiFeP o 4 . Here the confusion is quite common and immediately shows how much you can trust such a source.
Another major mistake is the opposition of a LiPo battery to a lithium-ion battery. Here are a few comparison options. The first is a general, associated with the misconception about the existence on the market of batteries with polymer electrolyte. The second, having a narrower application, which is usually voiced in the following form "lithium-polymer battery [talking about the body] is better / worse than LFP / LTO / NCA (substitute the right one)".
Here is a mix of body type and filling.
For example, on this link you can read about the LFP battery in a lithium-polymer format (prismatic case in this case).
This is another kind of twisting of facts for sale reasoning. This method is used for different types of batteries, but the LFP variant of a battery and a lithium-cobalt or NMC with a graphite cathode are most often compared. In articles on the Internet, both promotional and simply popular, you can find the ratio of full equivalent cycles in 2000 to 500 in favor of LFP and, as a result, a story about the significant superiority of the first.
There are some inaccuracies here. First, the majority of articles on lithium-cobalt are dated 2005–2006, while for LFP it is dated from 2012–2013. Cycle data is based on these articles. Nevertheless, the development did not stop and were equally active for all types of batteries and the gap is not so large in one and the same time interval. Secondly, the amount of energy that the battery will transmit over its life is not specified, and yet with equal sizes, the LFP has a smaller capacity.
As for the main advantage - a greater number of cycles, then if we take new research and compare production samples under equal conditions, the difference is not that dramatic. In total, it is 20-30% (800 cycles versus 1000 for 40 ° C, for example), which does not always justify the purchase of the same LFP, since less energy will be transferred due to the smaller voltage difference over the entire life cycle.
There are no sources with direct comparison, since the testing process itself is lengthy and expensive, complicated by contracts about not disclosing the names of participants, but comparing for a number of data it can be concluded that today there are similar characteristics for all lithium-ion batteries in terms of service life in all possible scenarios, including and simple storage. These data are given, for example, in sources 1 , 2 , 3 , 4 , 5 , 6 , 7 .
BU-206: Lithium-polymer: Substance or Hype?
Kazuo Murata, Shuichi Izuchi, Youetsu Yoshihisa "
A. Manuel Stephan, KS Nahm " Review on composite polymer electrolytes for lithium batteries. Polymer "
D. Golodnitskya, E. Straussc, E. Peleda and S. Greenbaum Review - On
My Lithium-Ion Batteries - Operation of Lithium-Ion Batteries
With this article I would like to clarify some points and conduct a kind of educational program.
Lithium polymer batteries
Right from the main point - there is no free lithium-polymer batteries in the technical sense of the word in the market. In the English-speaking world, this has already been sorted out, but in the post-Soviet space there are some costs in the terminology used by marketers. A small digression is not that they are not used in other regions, but at least there is an opportunity to check this information in their native language.
A bit of history
Any lithium-ion battery has 4 main components - two electrodes (anode and cathode), an electrolyte and a separator. All 4 elements have developed and are developing further. At the beginning of the research (1970s), two options were proposed for electrolyte — liquid or solid electrolyte. At that time, the solid electrolyte promised more prospects for operation - the electrolyte does not leak out if the casing is damaged, the element itself is more durable. The main drawback was and remains the high resistance of solid electrolyte, it negates the physical characteristics.
In fact, a decrease in the amount of resources allocated by companies to develop solid electrolytes occurred in the early 1990s, when Sony introduced a liquid electrolyte battery to the market. Sony itself in 1988 was confident in the future success of solid electrolyte.
Despite the orientation of the liquid electrolyte, companies have not stopped looking for alternatives. One of the options was the so-called hybrid electrolytes. In fact, they use a separator with small holes and the same liquid electrolyte. Although it feels dry to the touch, in fact the amount of electrolyte in it does not differ from that in a conventional battery. Both in principle and construction:

Schematic model of a lithium-ion battery with a LiCoO 2 cathode and graphite anode from Wikipedia in German.
These batteries are quite common, their commercial distribution began in the early 2000s, but physically and chemically they are the same lithium-ion batteries with liquid electrolyte and in general there are not very many of them.
What is on the market?
One way to classify batteries is its case. Today there are three popular packaging methods:
- Cylindrical cells
- Prismatic cells
- "Bag" or pouch-bag cells
The first type of battery is known for its use in laptops and Tesla cars (it uses the most common size of 18,650).
The second type is a modified cylindrical shape. Aluminum body, rectangle or square in cross section. Popular for stationary use and in transport.
The third type has a soft body and is not always equipped with a built-in protection system. In fact, a cheaper version of the prismatic cell. This type of battery is used in particular in mobile phones.
The last ones in the list are those “polymer” ones. They are so called for several reasons. The most arrogant way to marketers - the case of polymers, and therefore "polymer".
The second option is the use of a fine polymer separator. In fact, no different from a conventional lithium-ion battery.
The third option, which I have not met, is to give the name “polymer” based on the use of polymer elements as bases of cathodes, anodes, and other elements. Usually falls into a lot of batteries in a plastic case.
Terminology problems
When developing the concept, the idea was that the term “liquid electrolyte” was understood to mean a liquid or gel-like solution of lithium salt, while the term “solid electrolyte” (solid electrolyte) was a solid state of matter. Since there was a desire to sell what was promised but what is not, today, even among researchers, gel electrolyte is added to the list of "solid" electrolytes, although its characteristics are still more hybrid. Therefore, it is possible to meet the description in scientific papers “solid gel electrolyte”, which some scientists consider to be misleading.
The future of polymer electrolytes
Developments are underway and in the future, the appearance of batteries with a real polymer electrolyte is possible. However, as of 2015, laboratory samples of polymer electrolytes based on organic chemistry did not show any tangible progress, because at the date of publication of the article in the foreseeable future there will be no mass withdrawal from liquid electrolyte.
Problems with the name of the battery types
There are several different types of lithium-ion batteries on the market. They have various names that allow you to describe their characteristics in terms of capacity or safety. In general, the following types can be found:
- Lithium-cobalt with a cathode LiCoO 2 - the most capacious models have a graphite anode.
- Li-manganese oxide with cathode LiMn 2 O 4 , Li 2 MnO 3 or LMnO, the latter can act as simply lithium-manganese
- Lithium-nickel-manganese-cobalt-oxide or NMC with cathode LiNiMnCoO 2
- Lithium-iron-phosphate with a cathode LiFePO 4 (LFP)
- Lithium-nickel-cobalt-aluminum-oxide (NCA) with a cathode LiNiCoAlO 2
- Lithium-titanate-oxide (LTO) with anode of Li 4 Ti 5 O 12
Immediately, you can notice the unevenness of titles. Some are named after the cathode, some after the anode. And if in the first case, you can still try to guess with a high degree of probability that the anode will be graphite, then in the case of the name by the anode, it remains only to guess. Also today, developments are underway and, in principle, a battery with a LiFePO 4 cathode and Li 4 Ti 5 O 12 anode can be found on the market , i.e. lithium-iron-phosphate lithium-titanate, which in this system do not have a simple marketing name. By reference - a scientific article of 2013 with tests of such a battery .
The reason for the existence of such a large number of cathodes and anodes of batteries in different battery requirements. Somewhere need more security, and somewhere capacity or power. You can get an idea of the stored energy based on the fact that each type of cathode and anode has a different potential, as can be seen from the images below (the potential of metallic lithium is chosen as 0 V potential, the voltage difference is greater - more power, energy density depends on the number of atoms lithium):

General scheme with potentials from the University of Kiel. Source

Material from a 2013 article by Jiantie Xu, Shixue Dou, and others. Source

Another picture from the Purdue School of Engineering and Technology. A source
The general understanding of the causes can produce the following rough image communication potential elements and the possibility of metallization of lithium at very low discharge or thermal instability overcharge:

Pictures taken from lectures
most unsafe to operate on the market - the lithium-cobalt with a graphite anode, the safest - with the cathode LiFePO 4 and the anode Li 4 Ti 5 O 12 . Naturally, the presence of the BMS (Battery Management System) reduces the risks, but you should not neglect them, this system will not be able to prevent the same too strong discharge, which is critical for batteries with a graphite anode.
Common mistakes
Common mistakes
The most common and most common mistake is the opposition to the "ordinary lithium-ion battery." As can be seen above, there is simply no such thing as “ordinary”. And the difference in voltages can be very different for seemingly identical cathodes and the same for different sets of cathodes and anodes.
The second error, not so significant, related to the previous item, writing the material of the cathode LiFePO 4 as follows - LiFeP o 4 . Here the confusion is quite common and immediately shows how much you can trust such a source.
Another major mistake is the opposition of a LiPo battery to a lithium-ion battery. Here are a few comparison options. The first is a general, associated with the misconception about the existence on the market of batteries with polymer electrolyte. The second, having a narrower application, which is usually voiced in the following form "lithium-polymer battery [talking about the body] is better / worse than LFP / LTO / NCA (substitute the right one)".
Here is a mix of body type and filling.
For example, on this link you can read about the LFP battery in a lithium-polymer format (prismatic case in this case).
Battery A is more durable than Battery B
This is another kind of twisting of facts for sale reasoning. This method is used for different types of batteries, but the LFP variant of a battery and a lithium-cobalt or NMC with a graphite cathode are most often compared. In articles on the Internet, both promotional and simply popular, you can find the ratio of full equivalent cycles in 2000 to 500 in favor of LFP and, as a result, a story about the significant superiority of the first.
There are some inaccuracies here. First, the majority of articles on lithium-cobalt are dated 2005–2006, while for LFP it is dated from 2012–2013. Cycle data is based on these articles. Nevertheless, the development did not stop and were equally active for all types of batteries and the gap is not so large in one and the same time interval. Secondly, the amount of energy that the battery will transmit over its life is not specified, and yet with equal sizes, the LFP has a smaller capacity.
As for the main advantage - a greater number of cycles, then if we take new research and compare production samples under equal conditions, the difference is not that dramatic. In total, it is 20-30% (800 cycles versus 1000 for 40 ° C, for example), which does not always justify the purchase of the same LFP, since less energy will be transferred due to the smaller voltage difference over the entire life cycle.
There are no sources with direct comparison, since the testing process itself is lengthy and expensive, complicated by contracts about not disclosing the names of participants, but comparing for a number of data it can be concluded that today there are similar characteristics for all lithium-ion batteries in terms of service life in all possible scenarios, including and simple storage. These data are given, for example, in sources 1 , 2 , 3 , 4 , 5 , 6 , 7 .
Other sources
BU-206: Lithium-polymer: Substance or Hype?
Kazuo Murata, Shuichi Izuchi, Youetsu Yoshihisa "
A. Manuel Stephan, KS Nahm " Review on composite polymer electrolytes for lithium batteries. Polymer "
D. Golodnitskya, E. Straussc, E. Peleda and S. Greenbaum Review - On
My Lithium-Ion Batteries - Operation of Lithium-Ion Batteries
