The explosion and the global conspiracy: the story of the creation of lithium-ion batteries

Before you start reading, calculate how many devices with batteries are next to you within a radius of several meters. Surely you will see a smartphone, tablet, smart watch, fitness tracker, laptop, wireless mouse? All of these devices are equipped with lithium-ion batteries - their invention can be considered one of the most important events in the field of energy.
Lightweight, capacious and compact lithium-ion batteries contributed to the boom in portable electronics, the existence of which was previously impossible. Just gadgets over the past 30 years have made a fantastic technological leap, and modern lithium-ion batteries are almost no different from the first production models of the early 1990s. Who and how invented lithium-ion rechargeable batteries, what compositions are used in them, and is there a global conspiracy against “eternal” batteries? We are telling.
The legend of the first battery
Between the first attempt to produce electricity chemically and the creation of lithium-ion batteries, perhaps two millennia have passed. There is an unconfirmed guess that the first man-made galvanic element in the history of mankind was the “Baghdad battery”, found in 1936 near Baghdad by the archaeologist Wilhelm Koenig. The find, dated II-IV century BC. e., is a clay vessel in which there is a copper cylinder and an iron rod, the space between which could be filled with "electrolyte" - acid or alkali. Modern reconstruction of the find has shown that when the vessel is filled with lemon juice, it is possible to achieve a voltage of up to 0.4 volts.
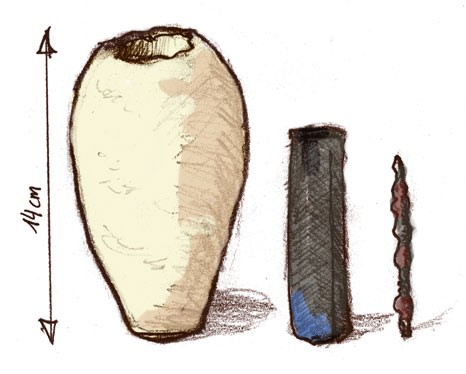
The Baghdad Battery is quite similar to a portable battery. Or a papyrus case? Source: Ironie / Wikimedia
Why could the “Baghdad battery” be used if a couple of thousand years remained before the discovery of electricity? Perhaps it was used for the accurate deposition of gold on figurines by the galvanization method - current and voltage from the “battery” are quite enough for this. However, this is only a theory, because the ancient peoples did not get any evidence of the use of electricity and this same “battery”: gilding was applied by amalgamation at that time, and the unusual vessel itself could just as well be a protected container for scrolls.
The Little Bang Theory
The Russian proverb “There would be no happiness, but misfortune helped” illustrates the course of work on lithium-ion batteries in the best possible way. Without one unexpected and unpleasant incident, the creation of new batteries could be delayed for several years.
Back in the 1970s, Briton Stanley Whittingham, who worked at Exxon Energy Company, used a titanium sulfide anode and a lithium cathode to create a rechargeable lithium battery. The first rechargeable lithium battery showed tolerable current and voltage indicators, it only exploded periodically and poisoned the people around it with gas: titanium disulfide released hydrogen sulfide upon contact with air, which is at least unpleasant to breathe, but at the most dangerous. In addition, titanium has always been very expensive at all times, and in the 1970s the price of titanium disulfide was about $ 1,000 per kilogram (the equivalent of $ 5,000 nowadays). Not to mention the fact that metallic lithium burns in air. So Exxon turned Whittingham's project away from sin.
In 1978, Koichi Mizushima, who defended his doctorate in physics, was researching at Tokyo University when he received an invitation from Oxford to join John Goodenough's group to search for new materials for battery anodes. This was a very promising project, since the potential of lithium power sources was already known, but they could not really manage to tame the capricious metal - recent Whittingham experiments showed that the start of mass production of the coveted lithium-ion batteries was still a long way off.
The experimental batteries used a lithium cathode and a sulfide anode. The superiority of sulfides over other materials in the anodes gave Mizushima and his colleagues a direction to search. Scientists ordered a sulphide furnace in their laboratory on site to experiment with different compounds faster. Work with the stove did not end very well: one day it exploded and caused a fire. The incident forced a team of researchers to reconsider their plans: perhaps sulfides, despite their effectiveness, were not the best choice. Scientists have shifted their attention towards oxides, which were much safer to synthesize.
After many tests with various metals, including iron and manganese, Mizushima found that lithium cobalt oxide shows the best results. Just use it not in the same way as Gudenaf’s team had supposed before - to look for not material that absorbs lithium ions, but material that most readily gives lithium ions. Cobalt was also better than others because it meets all safety requirements and also increases the cell voltage to 4 volts, that is, twice as much compared to earlier battery options.
The use of cobalt was the most important, but not the last step in creating lithium-ion batteries. Having dealt with one problem, scientists faced another: the current density was too low for the use of lithium-ion cells to be economically viable. And the team that made one breakthrough made the second one: when reducing the thickness of the electrodes to 100 microns, it was possible to increase the current strength to the level of other types of batteries, with double voltage and capacity.
First commercial steps
The story of the invention of lithium-ion batteries does not end there. Despite the discovery of Mizushima, the Gudenaf team did not yet have a sample ready for mass production. Due to the use of lithium metal in the cathode during the battery charge, lithium ions returned to the anode not in an even layer, but by dendrites - embossed chains, which, growing, caused a short circuit and fireworks.
In 1980, the Moroccan scientist Rachid Yazami discovered that graphite copes with the role of a cathode, and it is absolutely fireproof. The organic electrolytes existing at that time quickly decomposed on contact with graphite, so Yazami replaced them with solid electrolyte. Yazami's graphite cathode was inspired by the discovery of polymer conductivity by Professor Hideki Shirakawa, for which he received the Nobel Prize in Chemistry. And the Yazami graphite cathode is still used in most lithium-ion batteries.
Launch into production? And again no! Another 11 years passed, the researchers improved battery safety, increased voltage, experimented with different cathode materials before the first lithium-ion battery went on sale.
The commercial sample was developed by Sony and the Japanese chemical giant Asahi Kasei. It became the battery for the film amateur camcorder Sony CCD-TR1. It withstood 1000 charge cycles, and the residual capacity after such wear was four times higher than that of a similar type of nickel-cadmium battery.
Cobalt Stumbling Block
Prior to the discovery of Koichi Mizushima lithium-cobalt oxide, cobalt was not a much sought after metal. Its main deposits were discovered on the territory of Africa in the state now known as the Democratic Republic of the Congo. Congo is the largest supplier of cobalt - 54% of this metal is mined here. Due to political upheaval in the country in the 1970s, the price of cobalt soared by 2000%, but later returned to its previous values.
High demand creates high prices. Neither in the 1990s, nor in the 2000s, cobalt was one of the main metals on the planet. But what began with the popularization of smartphones in the 2010s! In 2000, demand for metal was approximately 2,700 tons per year. By 2010, when iPhone and Android smartphones were triumphantly marching around the planet, demand jumped to 25,000 tons and continued to grow year by year. Now the number of orders exceeds the volume of cobalt sold by 5 times. For reference: more than half of the cobalt mined in the world goes to the production of batteries.
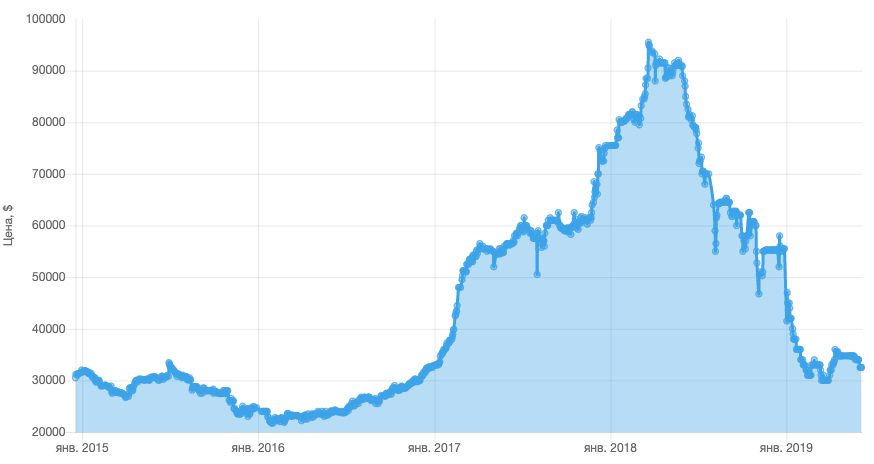
Cobalt price chart for the last 4 years. Comments are redundant. Source: Elec.ru
If in 2017 the price per ton of cobalt averaged $ 24,000, then since 2017 it has gone up steeply, in 2018 reaching a peak at around $ 95,500. Although smartphones use only 5-10 grams of cobalt, rising metal prices have affected the cost of the devices.
And this is one of the reasons why electric car manufacturers are concerned about the decrease in the share of cobalt in car batteries. For example, Tesla reduced the mass of scarce metal from 11 to 4.5 kg per machine, and in the future plans to find effective compounds without cobalt at all. By 2019, the price of cobalt rose abnormally and fell to the values of 2015, but battery developers have stepped up efforts to refuse or reduce the share of cobalt.
In traditional lithium-ion batteries, cobalt is about 60% of the total mass. The lithium-nickel-manganese composition used in automobiles comprises from 10% to 30% cobalt, depending on the desired characteristics of the battery. Lithium-nickel-aluminum composition is only 9%. However, these mixtures are not a complete replacement for lithium cobalt oxide.
Li-ion problems
To date, various types of lithium-ion batteries are the best batteries for most consumers. Capacious, powerful, compact and inexpensive, they still have serious drawbacks that limit the scope of use.
Fire hazard.For normal operation, a lithium-ion battery needs a power controller to prevent overcharging and overheating. Otherwise, the battery turns into a very flammable thing, striving to swell and explode in the heat or when charging from a poor-quality adapter. Explosion hazard is perhaps the main drawback of lithium-ion batteries. To increase the capacity inside the batteries, the arrangement is compacted, due to which even slight damage to the shell instantly leads to a fire. Everyone remembers the sensational story with the Samsung Galaxy Note 7, in which, due to the tightness inside the case, the battery shell rubbed over time, oxygen penetrated inside and the smartphone suddenly flashed. Since then, some airlines require that you carry lithium-ion batteries only in your carry-on baggage,
Depressurization is an explosion. Recharge is an explosion. The energy potential of lithium has to be paid with precautions
. Aging. Lithium-ion batteries are prone to aging, even if not used. Therefore, bought as a collector unpacked smartphone 10 years ago, for example, the very first iPhone, will hold a charge much less due to the very aging of the battery. By the way, the recommendations to keep the batteries charged up to half the capacity are justified - when fully charged during long storage, the battery loses its maximum capacity much faster.
Self-discharge.Accumulating energy in lithium-ion batteries and storing it for many years is a bad idea. In principle, all batteries lose their charge, but lithium-ion do this especially quickly. If NiMH cells lose 0.08–0.33% per month, then Li-Ion cells lose 2-3% per month. Thus, in a year the lithium-ion battery will lose a third of the charge, and after three years it will “sit down” to zero. In fairness, let’s say that nickel-cadmium batteries are still worse - 10% per month. But this is a completely different story.
Sensitivity to temperature.Cooling and overheating strongly affect the parameters of such a battery: +20 ° C degrees are considered the ideal ambient temperature for lithium-ion batteries, if it is reduced to +5 ° C, the battery will give the device 10% less energy. Cooling below zero takes tens of percent from the capacity and also affects the health of the battery: if you try to charge it, for example, from a power bank, a "memory effect" will appear, and the battery will irrevocably lose capacity due to the formation of lithium metal on the anode. At average winter Russian temperatures, the lithium-ion cell is not functional - leave the phone in January on the street for half an hour to verify this.
To cope with the problems described, scientists are experimenting with materials from anodes and cathodes. When replacing the composition of the electrodes, one big problem is replaced by smaller problems - fire safety leads to a decrease in the life cycle, and a high discharge current reduces the specific energy consumption. Therefore, the composition for the electrodes is selected depending on the application of the battery.
Who stole the revolution?
Every year on news feeds there are reports of another breakthrough in creating extremely capacious and enduring batteries - it seems like smartphones will work for a year without recharging, and charge in ten seconds. And where is the battery revolution that scientists promise everyone?
Often in such reports, journalists distort the facts, omitting any very important details. For example, a battery with instant charging can have a very low capacity, suitable only for powering a bedside alarm clock. Or the voltage does not reach one volt, although smartphones need 3.6 V. And to get a ticket to life, the battery needs to have a low cost and high fire safety. Unfortunately, the vast majority of developments were inferior in at least one parameter, because of which the "revolutionary" batteries never went beyond the laboratory.

In the late 00s, Toshiba experimented with rechargeable methanol fuel cells (refueling with methanol in the photo), but lithium-ion batteries were still more convenient. Source: Toshiba
And, of course, we leave aside the conspiracy theory "endless batteries are not profitable for manufacturers." Nowadays, batteries in consumer devices are irreplaceable (or rather, they can be changed, but difficult). 10-15 years ago, replacing a damaged battery in a mobile phone was simple, but then the power sources really lost much capacity in a year or two of active use. Modern lithium-ion batteries last longer than the average life cycle of the device. In smartphones, you can think about replacing the battery no earlier than after 500 charging cycles when it loses 10-15% of its capacity. And rather, the phone itself will lose relevance before the battery finally fails. That is, battery manufacturers do not earn on replacement, but on the sale of batteries for new devices.
Goodenough's team back in business
But what happened to the scientists of the John Goodenough group, who discovered the lithium-cobalt oxide and thereby gave life to efficient lithium-ion batteries?
In 2017, the 94-year-old Goodenough announced that, together with scientists from the University of Texas, he had developed a new type of solid state battery that could store 5-10 times more energy than previous lithium-ion batteries. For this, the electrodes were made of pure lithium and sodium. Promised and low price. But there are still no specifics and forecasts about the beginning of mass production. Given the long way between the opening of the Gudenaf group and the start of mass production of lithium-ion batteries, real samples can be expected in 8-10 years.
Koichi Mizushima continues research at Toshiba Research Consulting Corporation. “Looking back, I am surprised that no one before us had guessed to use such simple material as lithium cobalt oxide on the anode. By that time, many other oxides had been tried, so probably if it weren’t for us, someone else would have made this discovery within a few months, ”he said.

Koichi Mizushima with an award from the Royal Chemical Society of Great Britain received for participating in the creation of lithium-ion batteries. Source: Toshiba
History does not tolerate subjunctive moods, especially since Mr. Mizushima himself admits that a breakthrough in the creation of lithium-ion batteries was inevitable. But still it’s interesting to imagine what the world of mobile electronics would be like without compact and capacious batteries: laptops with a thickness of a few centimeters, huge smartphones that need to be charged twice a day, and no smart watches, fitness bracelets, action cameras, quadcopters and even electric vehicles. Every day, scientists around the world are bringing a new energy revolution closer, which will give us more powerful and more compact batteries, and with them incredible electronics, which we can only dream about.
