Opus about His Majesty Clay. Part Three - Polyurethane vs Space Cold
- Tutorial
Dedicated to all water-boat tourists, fishermen and sailors who succeeded or did not succeed (but hopefully succeed after reading the article) to seal the holes on their PVC vessels, because they are not the only Desmokol ...
Polyurethanes came into my life in early childhood, when I fervently proved to my mother that it is more profitable to buy expensive sneakers from Belkelme with polyurethane soles, rather than Lida shoes with tearing-down incomprehensible plastic (later, by the way, the “Lida” ones were fixed), simply because “ polyurethane is eternal. ” In principle, the way it was, the sneakers wore out to dust, and the sole, sole looked like on the day of purchase. The second “discovery” of this important class of polymers for me took place when I got acquainted with water tourism and such a thing as a hole in the “skin” of PVC. I still remember the commandment of the coach, ms Vyacheslav Antonovich Bazhansky - "Desmokol-created for kayaking, like a bird for the sky."
So, friends, today is your next, third article from the “glue” series. It is dedicated to polyurethanes.. If you want to know how to wash the mounting foam, how to firmly glue the punched fishing pvc boat and which seal retains elasticity at the temperature of liquid nitrogen - go under the cat, everything is there!

In a previous article, about cyanoacrylate superglue, I gave a curious picture. Now, in order not to duplicate, I hide it under a spoiler.
From this picture it can be seen that polyurethanes (PU) absolutely deservedly take first place among all other adhesives. To understand the reasons will help the plate below, with a description of the advantages and disadvantages of this type of adhesives.
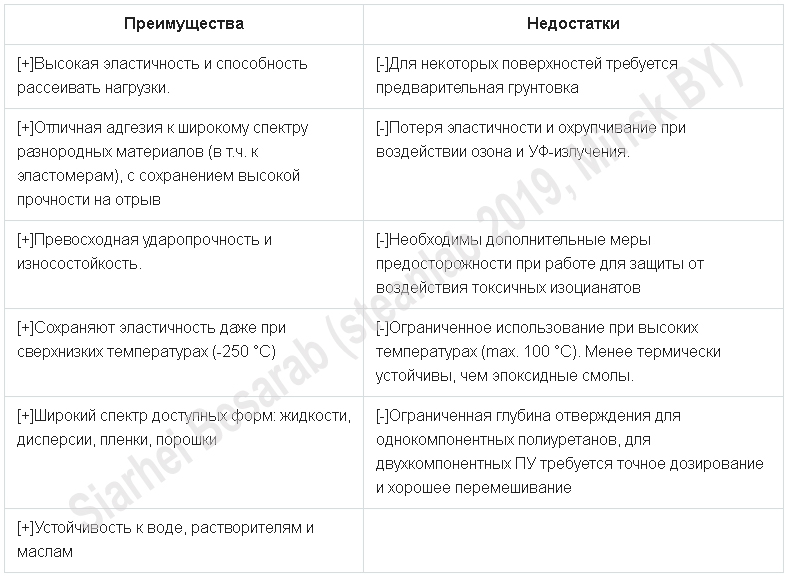
Some shortcomings and advantages of a more detailed interpretation will be given below, but for now, traditionally, facts from history.
Polyurethanes were first synthesized in Germany by Otto Bayer and his associates at IG Farbenindustrie in the late 1930s (the first patents date back to 1937).

The most talented, by the way, chemist. "Father of polyurethane" and founder of the now-famous chemical giant Bayer AG. His memory is immortalized in Germany by the annual Otto Bayer Prize - for outstanding scientific discoveries in the field of chemistry and biochemistry.
Uncle Otto’s first children were obtained by reacting an aliphatic diisocyanate with an aliphatic diamine or diol. In contrast to the difficult fate of cyanoacrylate, these materials almost immediately found commercial use and began to be sold under the trade names Irgamid U for plastics and Perlon U for synthetic fibers and bristles. After some time, it was found that isocyanates can be used to bind rubber to metal, which, in turn, has led to a surge of interest in the synthesis of urethane adhesives based on polyester diols. The first commercial product of this type was manufactured under the Polystal brand name.
That they didn’t stick with invented substances - from life rafts to tank equipment. In the early 1950s, urethane prepolymers were first used to join leather, wood, fabric, and rubber composites. A few years later, one of the first two-component urethane adhesives was proposed for use as an adhesive for metal-metal compounds. In 1957, the first thermoplastic polyurethane appeared, which was used as hot-melt adhesive (adhesive tapes), which was used for gluing sheet metal containers. New thermoplastic polyurethane adhesives began to appear in the period 1958-1959. In the early 1960s at the tire company BFGoodrichdeveloped thermoplastic polyester polyurethanes that glued leather and vinyl. In 1968, Goodyear introduced the first fiberglass structural adhesive used for truck hoods. Pressure-sensitive polyurethanes began to appear in the early 1970s. Under the same Goodyear brand, by 1978 advanced two-component automotive structural adhesives and water-based adhesives were on sale. In 1984, Bostik developed reactive hot melt adhesives. Well, then you already know ... Further, the evolution of adhesives and the latest trends in this area I have already described in one of my previous articles.
In general, almost a hundred years of detailed study and constant development of new compositions have led to the fact that today polyurethanes are one of the most famous universal polymer systems. This is confirmed by the variety of products that are manufactured using PU - elastic fibers and wear-resistant coatings, all kinds of foams, shock absorbers, adhesives and dozens and hundreds of products.

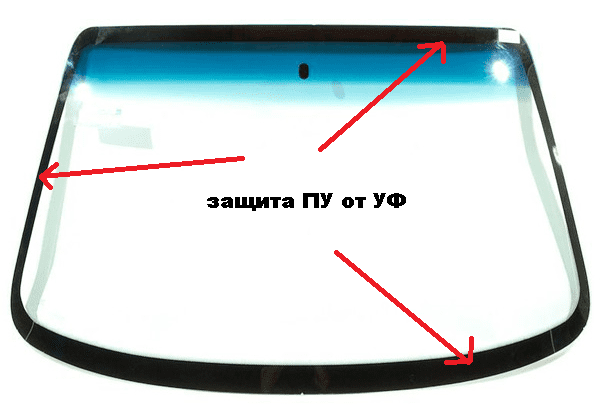
It is safe to say that the vast niche of polyurethane adhesives and sealants in the automotive industry (structural sealants, adhesives for plastics and fillers), the construction industry (joining wood, the production of chipboard and laminates, bonding large area plates of metals and composites, installation of glazing systems, high-strength floors, building sealants), shipbuilding (mounting adhesives, sealants) and the shoe industry (main adhesives) - busy and durable. For example, I’m not much mistaken if I say that all the windshields of cars are held on polyurethanes. By the way, the black stripe along the edge of the glass is not so much an aesthetic element as a protection for UV adhesive from polyurethane adhesive.
Here you can recall such a thing, quite distant from the same mounting foam, as lycra , it is also elastane, it is spandex - a synthetic fabric with unique elasticity (I immediately recall the advertisement “blah blah blah ... tights with lycra”). By the way, these fibers look no less attractive under polarized light:

Whatever one may say, it’s impossible to show pictures for a long time, anyway, to explain the reasons for many of the physical properties of polymers, one has to go down to the very basics, to the chemistry of high molecular weight compounds. What are we lower and do.
Polyurethanes are polymers containing urethane -NHCOO- groups in the polymer chain. They are most often obtained by the interaction of di- or tripolyisocyanate with a polyhydric alcohol ( polyol ). Since polyurethanes contain two types of monomers that polymerize one after another, they are classified as alternating copolymers. Both isocyanates and polyols (polyols) used to make polyurethanes contain, on average, two or more functional groups per molecule. The main building blocks of any polyurethane are shown schematically below.

In fairness, it is worth noting that often in "commercial" polyurethanes, other "extraneous" functional groups (ether, ester, amide or urea) are present in quantities much larger than the number of the same, urethane, groups.

By the way, a small remark. Polyurethanes are often referred to simply as urethanes, and you should not confuse them with a compound such as ethyl carbamate.(carbamic acid ethyl ester), which organic chemists often call urethane. Our "sabzhovye" urethanes have nothing to do with ethyl carbamate. In general, when they say "urethane glue", they usually mean an adhesive polymer obtained by isocyanate chemistry using reactions of isocyanates with active hydrogen compounds. But, if they are amiss, isocyanate reactions do not always lead to the formation of urethane bonds, just as there are methods for producing compounds with urethane bonds without the use of isocyanates. Therefore, in order to avoid confusion, it is believed that polyurethane adhesive is an adhesive that uses reactions involving the isocyanate group to polymerize and bond materials (—N = C = O, NCO).
As will be shown below, the properties of polyurethane largely depend on the types of isocyanates and polyols used to produce it. The long flexible segments introduced by the polyol provide a soft, flexible polymer. Large quantities of crosslinking give tough polymers. Long chains and weak crosslinking give a polymer that is very elastic, short chains with a lot of crosslinking give a solid polymer. In some respects, a piece of polyurethane can be thought of as one giant molecule. One of the consequences of this is that typical polyurethanes do not soften and do not melt when heated, they are thermosetting. This is a rather important property for glue, which claims to be the pedestal of the world of structural adhesives.
The correct structural adhesive (adhesive) must necessarily have molecules with three functional groups in order to form a three-dimensional structure with covalent bonds (otherwise, a regular thermoplast would be obtained). Therefore, the concentration of such molecules is decisive in the formation of a “working” adhesive structure (= the number of cross-links, which are described below).
If you recall the tablet “what better to glue”, which I showed in the first article:

you can see that polyurethane adhesives are best suited for bonding together elastomers / fabrics and composites / polymers with glass and ceramic / wood / metal. Those. The main use of polyurethanes is to bind plastics, which are difficult to bind, usually with dissimilar material or with metals. The following reasons can be mentioned, due to which PUs are excellent adhesives:
1. PUs effectively wet the surface of most materials (although for materials with low surface energy, again we recall polyethylene or polypropylene, to increase wetting it is necessary to use primers or adhesives with a special additive-wetting agent),
2. PU easily form hydrogen bonds with the surface of the material,
3. The small molecular size of PUs allows them to penetrate into porous and layered materials, wetting the fibers
4. PUs form covalent bonds with materials that have active hydrogen atoms (polar surface). The mechanism of formation of such a covalent bond of polyurethane adhesive with a polar surface is shown in the picture:

Polyurethanes are spatially a segmented polymer consisting of soft segments and hard segments (random or (AB) block polymer). The soft segment consists of a long chain polyol, for example, polyester or polyetherdiol. The rigid segment is formed of a polyisocyanate and a short chain extension of polyol or diamine.
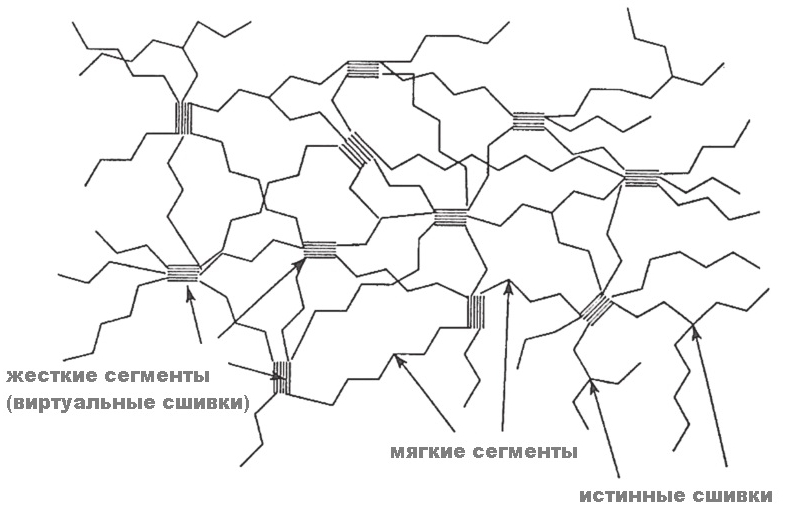
Due to the thermodynamic incompatibility of the two segments, phase separation occurs, leading to the formation of domains or microdomains inside the polymer. It is the presence of these microdomains that forms the unique, inherent only polyurethanes, viscoelastic behavior. It is the two-phase morphology of PU that is required by impact strength and a wide range of physical properties. Hard domains strengthen the polymer and act as crosslinking centers. Ultimately, virtual crosslinking (pseudo-crosslinking) is formed. Thanks to this structure, unusual material properties are formed (=, unlike most polymers, there are two temperature transitions, the first low-temperature (-20 ° C) due to the glass transition of the soft segment and the second high-temperature (> 80 ° C) associated with thermal dissociation of microdomains of the hard segment). Those. microdomains are what what polyurethane makes polyurethane (and there are no hydrogen bonds there, although they were often tried to attribute the laurels of the creators of the unique properties of PU). The uniqueness is visible from the plate, -240 ° C is almost the temperature of absolute zero temperature (−273.15 ° C), and here some material can also afford to stretch ...

I voiced the definition of structural adhesive, now I will also announce that polyurethanes are actively competing for the title of the main structural adhesive with epoxides and acrylics. The figure shows an approximate comparison of the main structural adhesives in terms of tensile elongation / shear modulus / tensile strength.
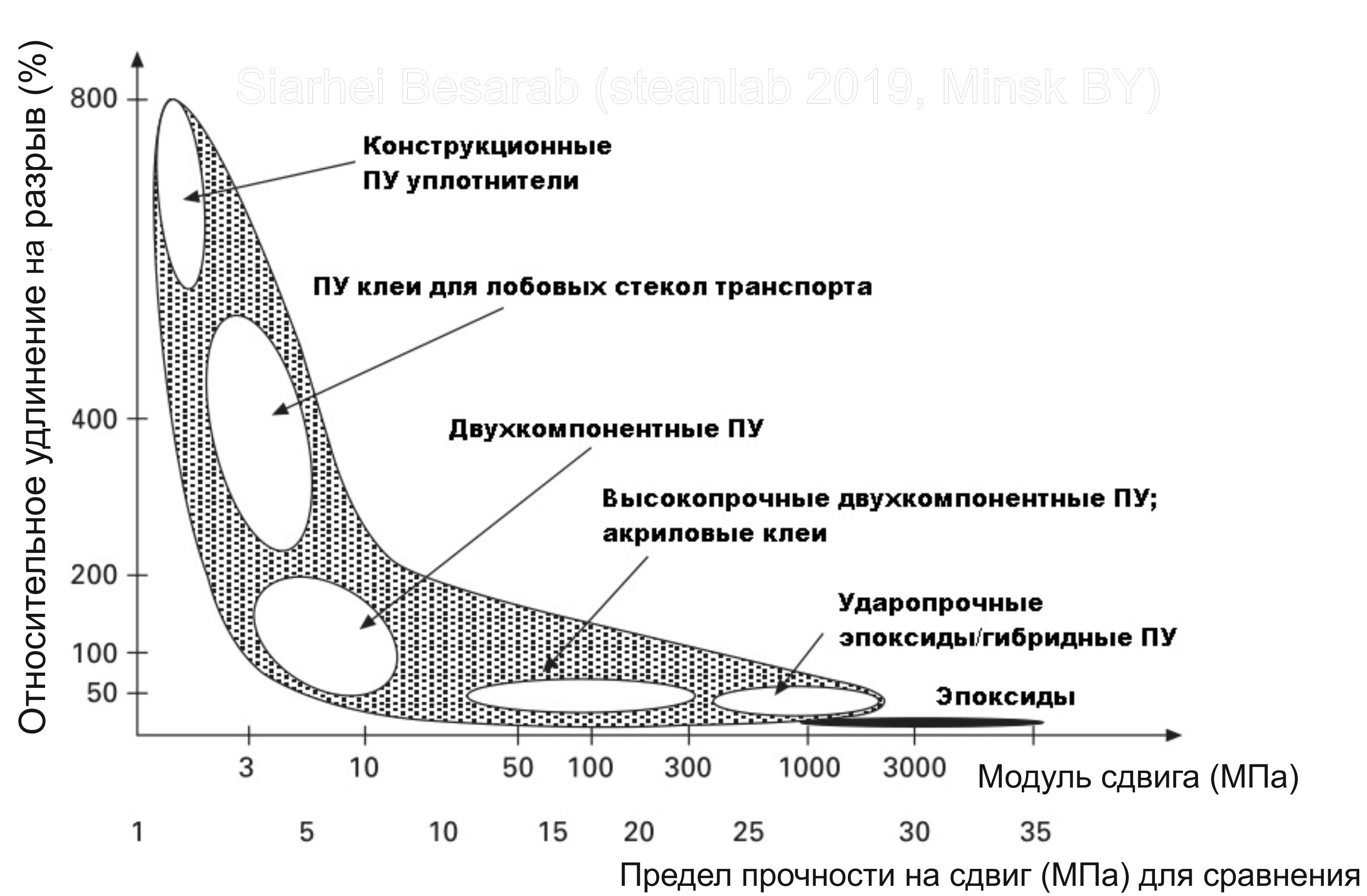
It is these glue properties, in fact, that are the key to understanding applicability in the context of a specific technical task. The shear modulus characterizes the stiffness of the bonds bonded to each other, the relative elongation at break shows whether it is possible to associate substrates of different thermal elongation with each other with an extension of the bonding line. The shear strength increases linearly with the shear modulus. As follows from the graph, polyurethanes are “ripe everywhere” and therefore are the most versatile adhesive. An important advantage is that their properties can be controlled in a very wide range - from very soft and elastic, rubber-like, to very hard and hard.
Now is the time to move on to classifying the world of polyurethanes. The following table is intended to help:

A new concept is the so-called "Blocked isocyanates." Blocking or masking of isocyanates is a reaction of isocyanate groups with a compound that prevents the interaction of the isocyanate with the polar surface at room temperature, but allows this reaction to occur at elevated temperatures. In addition, blocking allows solving the problem of hypersensitivity to humidity and creating aqueous dispersions or dispersions of isocyanate in polyhydric alcohol. Thanks to these capabilities, dozens of adhesive compositions have been developed for coating / laminating fabrics, adhesives for tire cords, etc., which is not possible using traditional components.
Polyurethane adhesives are divided into two main groups according to different curing mechanisms:
1. One-component adhesives are usually moisture cured.
2. Two-component adhesives are mixtures of resins and hardeners.
One-component polyurethane adhesives depend on air humidity and release isocyanates in various amounts during the curing cycle (by the way, I recommend walls / crevices, etc. when using mounting foam, moisten copiously with water, well, or alcohol, if you don't mind). Curing takes place rather slowly, the adhesive sets in 20-30 minutes and completely cures in 3-7 days, depending on the composition. Isocyanates, although not fatal in reasonable amounts, are nevertheless sufficiently strong irritating agent / skinsensitizer , so that the standard wish that is present in all of my last “polymer” articles is “an insulating mask with a cartridge for“ gases / vapors and / or supply and exhaust ventilation ”. And if in case of gluing a small object it is difficult to get a dangerous dose of isocyanate, then in the case of foaming the walls of acar in someone else's garage - it is quite possible. That the problem exists is also indicated by the concern of the EPA (United States Environmental Protection Agency), which issued a special postcard with safety tips for insulating with polyurethane aerosol foam (SPF) under this brand.

As for the most common components of this group of adhesives, the following can be mentioned:
Hydroxyl polyurethanes are thermoplastics with a content of hydroxyl groups of 0.5-1.0% and are obtained by the reaction of diphenylmethane-4-4-diisocyanate (MDI) with polyetherdiols. Thermoplastic polyurethanesreceive when hydroxylated polyurethanes are mixed with polyisocyanates. Curing occurs at room temperature. A process involving such an adhesive can be called differently: thermal bonding, high temperature vulcanization, reaction hot melt bonding, melt bonding, etc. The difference from the polymers that are used in traditional glue guns is that TPU is melted only for ease of application, the final properties of the glue line are formed in the process of classical curing with air moisture at room temperature. Hot glue from the gun immediately forms an adhesive joint after cooling. When emulsifying linear PU in water, polyurethane dispersion adhesives are obtained , which are actively used in the packaging and textile industries.
Unlike single-component, two-component polyurethane adhesives consist of low molecular weight polyisocyanates or prepolymers cured with low molecular weight polyols or polyamines. Despite the change in the method of curing, these PUs also sin the release of isocyanates, but in smaller quantities. A distinctive feature is the significantly reduced time required for the adhesive strength to acquire maximum strength. It is the two-component PUs used in the automotive industry for gluing metals with plastics, for foaming textile coatings, for gluing PVC film with wood in the furniture industry, etc.
In general, one- and two-component PUs cure well at room temperature (with the exception of the already mentioned hot-melt ones), but it is worth noting that heating the joints and seams accelerates polymer cross-linking. More clarity on the change in the strength of the adhesive joint, depending on the curing speed of different polyurethanes can give a picture:
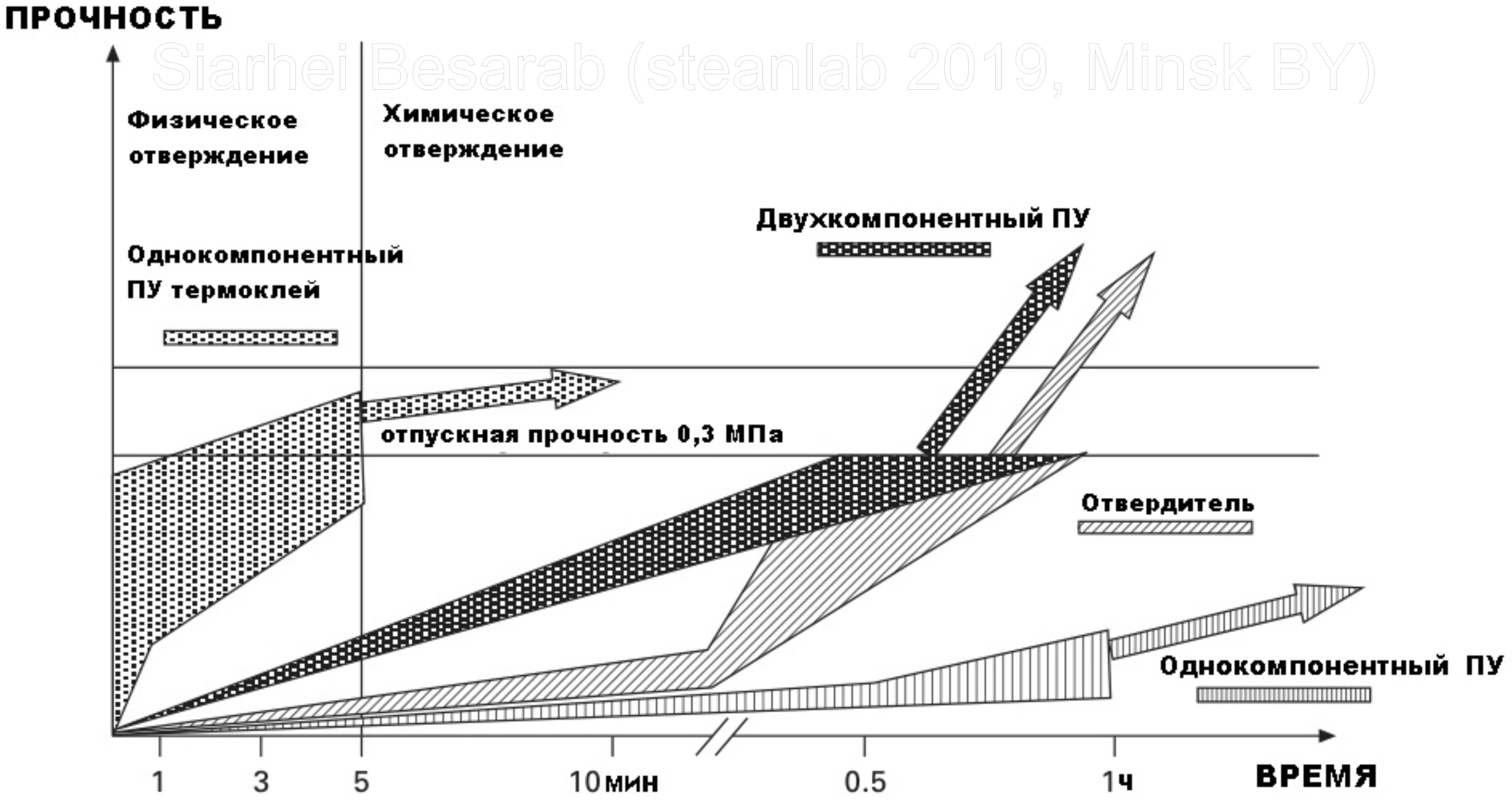
For binding, a figure of 0.3 MPa is given, since this is exactly the level to which polymers are oriented when developing the composition of glue for windshields. It can be seen from the graph that slow curing requires a longer fixation time to achieve the desired strength. Therefore, the glass is heated, thereby reducing the time required to set the required strength characteristics to 1 hour instead of 24 hours. However, it is not so simple as it would seem, because if the glue cools too quickly, it can eventually lead to delamination (an increase in cohesion occurs faster than the return of adhesive properties), and cause cracking of the glue under changing loads from air currents.
Separately, a few words about the preparation of the surface before gluing. The main purpose of surface treatment is the removal of any weak surface boundary layer on the substrate and / or the use of primers. The methods traditional for PU are mechanical treatment (“wipe with sandpaper") and degreasing of the surface with a solvent. Isocyanates themselves, which react with polar groups on the surface and promote binding, or silanes, are often used as primers. Silanes are usually used for glass (maximum efficiency - alkoxysilane primer binds to silanol groups on the glass surface, and the amino, mercapto or epoxy group of the same silane is attached to the isocyanate group of glue), fiber composites, mineral fillers, and cementitious surfaces. Epoxy silanes are also added to polyurethane adhesives to improve water resistance. Primers based on organic metal complexes — organotitanates, organozirconates, and some chromium complexes — are also known.
At the end of the story there are several thematic spoilers with a “practical life”.
Addition: at the request of APLe, I am attaching a list of foreign polyurethane adhesives.
Published earlier:
Opus about His Majesty Clay. Part One -
Introductory Opus about His Majesty Clay. Part Two - Viva, Cyanoacrylate! Viva, super glue
Opusa about His Majesty Clay. Part four - silicones.
Whether the next article will depend on the Habr community , for subj .

Polyurethanes came into my life in early childhood, when I fervently proved to my mother that it is more profitable to buy expensive sneakers from Belkelme with polyurethane soles, rather than Lida shoes with tearing-down incomprehensible plastic (later, by the way, the “Lida” ones were fixed), simply because “ polyurethane is eternal. ” In principle, the way it was, the sneakers wore out to dust, and the sole, sole looked like on the day of purchase. The second “discovery” of this important class of polymers for me took place when I got acquainted with water tourism and such a thing as a hole in the “skin” of PVC. I still remember the commandment of the coach, ms Vyacheslav Antonovich Bazhansky - "Desmokol-created for kayaking, like a bird for the sky."
So, friends, today is your next, third article from the “glue” series. It is dedicated to polyurethanes.. If you want to know how to wash the mounting foam, how to firmly glue the punched fishing pvc boat and which seal retains elasticity at the temperature of liquid nitrogen - go under the cat, everything is there!

In a previous article, about cyanoacrylate superglue, I gave a curious picture. Now, in order not to duplicate, I hide it under a spoiler.
Adhesive Market Distribution
From this picture it can be seen that polyurethanes (PU) absolutely deservedly take first place among all other adhesives. To understand the reasons will help the plate below, with a description of the advantages and disadvantages of this type of adhesives.

Some shortcomings and advantages of a more detailed interpretation will be given below, but for now, traditionally, facts from history.
Polyurethanes were first synthesized in Germany by Otto Bayer and his associates at IG Farbenindustrie in the late 1930s (the first patents date back to 1937).

The most talented, by the way, chemist. "Father of polyurethane" and founder of the now-famous chemical giant Bayer AG. His memory is immortalized in Germany by the annual Otto Bayer Prize - for outstanding scientific discoveries in the field of chemistry and biochemistry.
Uncle Otto’s first children were obtained by reacting an aliphatic diisocyanate with an aliphatic diamine or diol. In contrast to the difficult fate of cyanoacrylate, these materials almost immediately found commercial use and began to be sold under the trade names Irgamid U for plastics and Perlon U for synthetic fibers and bristles. After some time, it was found that isocyanates can be used to bind rubber to metal, which, in turn, has led to a surge of interest in the synthesis of urethane adhesives based on polyester diols. The first commercial product of this type was manufactured under the Polystal brand name.
That they didn’t stick with invented substances - from life rafts to tank equipment. In the early 1950s, urethane prepolymers were first used to join leather, wood, fabric, and rubber composites. A few years later, one of the first two-component urethane adhesives was proposed for use as an adhesive for metal-metal compounds. In 1957, the first thermoplastic polyurethane appeared, which was used as hot-melt adhesive (adhesive tapes), which was used for gluing sheet metal containers. New thermoplastic polyurethane adhesives began to appear in the period 1958-1959. In the early 1960s at the tire company BFGoodrichdeveloped thermoplastic polyester polyurethanes that glued leather and vinyl. In 1968, Goodyear introduced the first fiberglass structural adhesive used for truck hoods. Pressure-sensitive polyurethanes began to appear in the early 1970s. Under the same Goodyear brand, by 1978 advanced two-component automotive structural adhesives and water-based adhesives were on sale. In 1984, Bostik developed reactive hot melt adhesives. Well, then you already know ... Further, the evolution of adhesives and the latest trends in this area I have already described in one of my previous articles.
In general, almost a hundred years of detailed study and constant development of new compositions have led to the fact that today polyurethanes are one of the most famous universal polymer systems. This is confirmed by the variety of products that are manufactured using PU - elastic fibers and wear-resistant coatings, all kinds of foams, shock absorbers, adhesives and dozens and hundreds of products.

It is safe to say that the vast niche of polyurethane adhesives and sealants in the automotive industry (structural sealants, adhesives for plastics and fillers), the construction industry (joining wood, the production of chipboard and laminates, bonding large area plates of metals and composites, installation of glazing systems, high-strength floors, building sealants), shipbuilding (mounting adhesives, sealants) and the shoe industry (main adhesives) - busy and durable. For example, I’m not much mistaken if I say that all the windshields of cars are held on polyurethanes. By the way, the black stripe along the edge of the glass is not so much an aesthetic element as a protection for UV adhesive from polyurethane adhesive.

Here you can recall such a thing, quite distant from the same mounting foam, as lycra , it is also elastane, it is spandex - a synthetic fabric with unique elasticity (I immediately recall the advertisement “blah blah blah ... tights with lycra”). By the way, these fibers look no less attractive under polarized light:

And so it looks in public. as opposed to pantyhose

The reason for this popularity?
Whatever one may say, it’s impossible to show pictures for a long time, anyway, to explain the reasons for many of the physical properties of polymers, one has to go down to the very basics, to the chemistry of high molecular weight compounds. What are we lower and do.
Polyurethanes are polymers containing urethane -NHCOO- groups in the polymer chain. They are most often obtained by the interaction of di- or tripolyisocyanate with a polyhydric alcohol ( polyol ). Since polyurethanes contain two types of monomers that polymerize one after another, they are classified as alternating copolymers. Both isocyanates and polyols (polyols) used to make polyurethanes contain, on average, two or more functional groups per molecule. The main building blocks of any polyurethane are shown schematically below.

In fairness, it is worth noting that often in "commercial" polyurethanes, other "extraneous" functional groups (ether, ester, amide or urea) are present in quantities much larger than the number of the same, urethane, groups.

By the way, a small remark. Polyurethanes are often referred to simply as urethanes, and you should not confuse them with a compound such as ethyl carbamate.(carbamic acid ethyl ester), which organic chemists often call urethane. Our "sabzhovye" urethanes have nothing to do with ethyl carbamate. In general, when they say "urethane glue", they usually mean an adhesive polymer obtained by isocyanate chemistry using reactions of isocyanates with active hydrogen compounds. But, if they are amiss, isocyanate reactions do not always lead to the formation of urethane bonds, just as there are methods for producing compounds with urethane bonds without the use of isocyanates. Therefore, in order to avoid confusion, it is believed that polyurethane adhesive is an adhesive that uses reactions involving the isocyanate group to polymerize and bond materials (—N = C = O, NCO).
As will be shown below, the properties of polyurethane largely depend on the types of isocyanates and polyols used to produce it. The long flexible segments introduced by the polyol provide a soft, flexible polymer. Large quantities of crosslinking give tough polymers. Long chains and weak crosslinking give a polymer that is very elastic, short chains with a lot of crosslinking give a solid polymer. In some respects, a piece of polyurethane can be thought of as one giant molecule. One of the consequences of this is that typical polyurethanes do not soften and do not melt when heated, they are thermosetting. This is a rather important property for glue, which claims to be the pedestal of the world of structural adhesives.
Structural adhesive is an adhesive used to transfer the necessary loads between adhesive surfaces in complex structures exposed to the operating environment.
The correct structural adhesive (adhesive) must necessarily have molecules with three functional groups in order to form a three-dimensional structure with covalent bonds (otherwise, a regular thermoplast would be obtained). Therefore, the concentration of such molecules is decisive in the formation of a “working” adhesive structure (= the number of cross-links, which are described below).
If you recall the tablet “what better to glue”, which I showed in the first article:

you can see that polyurethane adhesives are best suited for bonding together elastomers / fabrics and composites / polymers with glass and ceramic / wood / metal. Those. The main use of polyurethanes is to bind plastics, which are difficult to bind, usually with dissimilar material or with metals. The following reasons can be mentioned, due to which PUs are excellent adhesives:
1. PUs effectively wet the surface of most materials (although for materials with low surface energy, again we recall polyethylene or polypropylene, to increase wetting it is necessary to use primers or adhesives with a special additive-wetting agent),
2. PU easily form hydrogen bonds with the surface of the material,
3. The small molecular size of PUs allows them to penetrate into porous and layered materials, wetting the fibers
4. PUs form covalent bonds with materials that have active hydrogen atoms (polar surface). The mechanism of formation of such a covalent bond of polyurethane adhesive with a polar surface is shown in the picture:

Polyurethanes are spatially a segmented polymer consisting of soft segments and hard segments (random or (AB) block polymer). The soft segment consists of a long chain polyol, for example, polyester or polyetherdiol. The rigid segment is formed of a polyisocyanate and a short chain extension of polyol or diamine.

Due to the thermodynamic incompatibility of the two segments, phase separation occurs, leading to the formation of domains or microdomains inside the polymer. It is the presence of these microdomains that forms the unique, inherent only polyurethanes, viscoelastic behavior. It is the two-phase morphology of PU that is required by impact strength and a wide range of physical properties. Hard domains strengthen the polymer and act as crosslinking centers. Ultimately, virtual crosslinking (pseudo-crosslinking) is formed. Thanks to this structure, unusual material properties are formed (=, unlike most polymers, there are two temperature transitions, the first low-temperature (-20 ° C) due to the glass transition of the soft segment and the second high-temperature (> 80 ° C) associated with thermal dissociation of microdomains of the hard segment). Those. microdomains are what what polyurethane makes polyurethane (and there are no hydrogen bonds there, although they were often tried to attribute the laurels of the creators of the unique properties of PU). The uniqueness is visible from the plate, -240 ° C is almost the temperature of absolute zero temperature (−273.15 ° C), and here some material can also afford to stretch ...

I voiced the definition of structural adhesive, now I will also announce that polyurethanes are actively competing for the title of the main structural adhesive with epoxides and acrylics. The figure shows an approximate comparison of the main structural adhesives in terms of tensile elongation / shear modulus / tensile strength.

It is these glue properties, in fact, that are the key to understanding applicability in the context of a specific technical task. The shear modulus characterizes the stiffness of the bonds bonded to each other, the relative elongation at break shows whether it is possible to associate substrates of different thermal elongation with each other with an extension of the bonding line. The shear strength increases linearly with the shear modulus. As follows from the graph, polyurethanes are “ripe everywhere” and therefore are the most versatile adhesive. An important advantage is that their properties can be controlled in a very wide range - from very soft and elastic, rubber-like, to very hard and hard.
Now is the time to move on to classifying the world of polyurethanes. The following table is intended to help:

A new concept is the so-called "Blocked isocyanates." Blocking or masking of isocyanates is a reaction of isocyanate groups with a compound that prevents the interaction of the isocyanate with the polar surface at room temperature, but allows this reaction to occur at elevated temperatures. In addition, blocking allows solving the problem of hypersensitivity to humidity and creating aqueous dispersions or dispersions of isocyanate in polyhydric alcohol. Thanks to these capabilities, dozens of adhesive compositions have been developed for coating / laminating fabrics, adhesives for tire cords, etc., which is not possible using traditional components.
One and two component adhesives
Polyurethane adhesives are divided into two main groups according to different curing mechanisms:
1. One-component adhesives are usually moisture cured.
2. Two-component adhesives are mixtures of resins and hardeners.
One-component polyurethane adhesives depend on air humidity and release isocyanates in various amounts during the curing cycle (by the way, I recommend walls / crevices, etc. when using mounting foam, moisten copiously with water, well, or alcohol, if you don't mind). Curing takes place rather slowly, the adhesive sets in 20-30 minutes and completely cures in 3-7 days, depending on the composition. Isocyanates, although not fatal in reasonable amounts, are nevertheless sufficiently strong irritating agent / skinsensitizer , so that the standard wish that is present in all of my last “polymer” articles is “an insulating mask with a cartridge for“ gases / vapors and / or supply and exhaust ventilation ”. And if in case of gluing a small object it is difficult to get a dangerous dose of isocyanate, then in the case of foaming the walls of a

As for the most common components of this group of adhesives, the following can be mentioned:
Hydroxyl polyurethanes are thermoplastics with a content of hydroxyl groups of 0.5-1.0% and are obtained by the reaction of diphenylmethane-4-4-diisocyanate (MDI) with polyetherdiols. Thermoplastic polyurethanesreceive when hydroxylated polyurethanes are mixed with polyisocyanates. Curing occurs at room temperature. A process involving such an adhesive can be called differently: thermal bonding, high temperature vulcanization, reaction hot melt bonding, melt bonding, etc. The difference from the polymers that are used in traditional glue guns is that TPU is melted only for ease of application, the final properties of the glue line are formed in the process of classical curing with air moisture at room temperature. Hot glue from the gun immediately forms an adhesive joint after cooling. When emulsifying linear PU in water, polyurethane dispersion adhesives are obtained , which are actively used in the packaging and textile industries.
Unlike single-component, two-component polyurethane adhesives consist of low molecular weight polyisocyanates or prepolymers cured with low molecular weight polyols or polyamines. Despite the change in the method of curing, these PUs also sin the release of isocyanates, but in smaller quantities. A distinctive feature is the significantly reduced time required for the adhesive strength to acquire maximum strength. It is the two-component PUs used in the automotive industry for gluing metals with plastics, for foaming textile coatings, for gluing PVC film with wood in the furniture industry, etc.
In general, one- and two-component PUs cure well at room temperature (with the exception of the already mentioned hot-melt ones), but it is worth noting that heating the joints and seams accelerates polymer cross-linking. More clarity on the change in the strength of the adhesive joint, depending on the curing speed of different polyurethanes can give a picture:

For binding, a figure of 0.3 MPa is given, since this is exactly the level to which polymers are oriented when developing the composition of glue for windshields. It can be seen from the graph that slow curing requires a longer fixation time to achieve the desired strength. Therefore, the glass is heated, thereby reducing the time required to set the required strength characteristics to 1 hour instead of 24 hours. However, it is not so simple as it would seem, because if the glue cools too quickly, it can eventually lead to delamination (an increase in cohesion occurs faster than the return of adhesive properties), and cause cracking of the glue under changing loads from air currents.
Separately, a few words about the preparation of the surface before gluing. The main purpose of surface treatment is the removal of any weak surface boundary layer on the substrate and / or the use of primers. The methods traditional for PU are mechanical treatment (“wipe with sandpaper") and degreasing of the surface with a solvent. Isocyanates themselves, which react with polar groups on the surface and promote binding, or silanes, are often used as primers. Silanes are usually used for glass (maximum efficiency - alkoxysilane primer binds to silanol groups on the glass surface, and the amino, mercapto or epoxy group of the same silane is attached to the isocyanate group of glue), fiber composites, mineral fillers, and cementitious surfaces. Epoxy silanes are also added to polyurethane adhesives to improve water resistance. Primers based on organic metal complexes — organotitanates, organozirconates, and some chromium complexes — are also known.
At the end of the story there are several thematic spoilers with a “practical life”.
Practical Lesson # 1 - Foam
“After a review, an idea immediately arose” and it’s better
After watching a video about a man, an ax and a cylinder of polyurethane foam, my first thought was “what is he going to wash it off with?” So this spoiler is intended for humanists who were struck by a similar thought (well, for those who, for example, well-wishers “foamed the car”, see KDPV).
Cured polyurethane in the form of polyurethane foam, in fact, is a thermosetting polymer, and therefore it does not melt succeed, as well as dissolve. “I’ve been dissolving acetone for 100,500 years and it’s normal,” the meticulous reader will say, and he will be wrong. Because this polymer does not dissolve acetone, it penetrates the internal porous structure, causes the frozen foam to swell and become softer. By repeating this procedure cyclically, you can destroy the cured three-dimensional frame.
In general, the resistance of PU foam to a solvent depends on the ratio of urethane and urea groups. The more urea groups, the greater the number of strong urea microdomains and the more difficult the dissolution process will be. In any case, a polar aprotic solvent such as N-methyl-2-pyrrolidone (NMP), N, N-dimethylethylene urea, DMF or DMSO is needed. If the foam does not go away, you can add a lithium salt, for example, LiCl, to the solvent. The picture below shows the resistance of various polyurethane foams to certain solvents:

As can be seen, DMSO has shown excellent dissolving ability for almost all types of urethane raw materials / foams, except for the most resistant of cured substrates. So, if you squeeze - still have to go to the pharmacy for " Dimexidum " th
After watching a video about a man, an ax and a cylinder of polyurethane foam, my first thought was “what is he going to wash it off with?” So this spoiler is intended for humanists who were struck by a similar thought (well, for those who, for example, well-wishers “foamed the car”, see KDPV).
Cured polyurethane in the form of polyurethane foam, in fact, is a thermosetting polymer, and therefore it does not melt succeed, as well as dissolve. “I’ve been dissolving acetone for 100,500 years and it’s normal,” the meticulous reader will say, and he will be wrong. Because this polymer does not dissolve acetone, it penetrates the internal porous structure, causes the frozen foam to swell and become softer. By repeating this procedure cyclically, you can destroy the cured three-dimensional frame.
In general, the resistance of PU foam to a solvent depends on the ratio of urethane and urea groups. The more urea groups, the greater the number of strong urea microdomains and the more difficult the dissolution process will be. In any case, a polar aprotic solvent such as N-methyl-2-pyrrolidone (NMP), N, N-dimethylethylene urea, DMF or DMSO is needed. If the foam does not go away, you can add a lithium salt, for example, LiCl, to the solvent. The picture below shows the resistance of various polyurethane foams to certain solvents:

As can be seen, DMSO has shown excellent dissolving ability for almost all types of urethane raw materials / foams, except for the most resistant of cured substrates. So, if you squeeze - still have to go to the pharmacy for " Dimexidum " th
Practical lesson №2, or Serezha’s bags
I would write about polyurethane glue, if only because all my packing bags were glued with it ...
About boat repair
It would be wrong if, speaking about PU, I would not mention my experience of gluing PVC and did not describe the algorithm of this process. In order not to torment the keyboard again, I just copied a couple of pieces from the water trip report.

The glue used is the cheapest shoe that can be found in our area. The national synonyms of polyurethane glue are Desmacol, Desmacol + Desmodure Hardener, Desmocol, Uranium, Bostic, 88, Black Tigrostik, Conveyor Belt Adhesive, UR600, Sar ”,“ Bonikol ”,“ Super-HH + hardener of enamel Izur-021 / Diur / Diur-extra / Izur-022 ”, Cosmofen,“ Cosmofen ”. Hardener - polyisocyanate, which will work equally well with any polyurethane raw material.
About pressure bags .
Do you like hermetic bags as I like them? It is likely that for every beginner / “one-time” water-tourist, right at that moment somewhere elder comrades say, “OK, don’t get loaded, instead of the shema take chalks from thick polyethylene for pickling cabbage”. For me it’s enough to go to the rafting once to understand what kind of rubbish this is, bags for cabbage, and why it’s better to buy even the cheapest, but still PVC pressure seal. Prices still bite, but in fact, after all, an ordinary bag is made of a PVC banner for advertising. I walked around the sporting goods stores, thought so, thought, and finally matured. After one of the events, we managed to beg the organizers for a banner (“the tables will be set to protect against acids”) and proceed with gluing. Plus, the work of the Master @ divecasper really warmed me upwith its germobaules and sealing bags. Looking at the photo - a lot of ideas appeared. For example, what are these works worth:

or

As a result, I have matured for the practical application of accumulated knowledge. Surprisingly, the first pancake did not come out lumpy. The glued bags, although they were unprepossessing and made of relatively thin PVC, passed two water trips quite confidently, moreover one in the deep off-season, along our “Pre-Karelian training” river Molchad. By the way, they still go. Their photo, unfortunately, has not been preserved. Then more experienced, I used “acetone”, “PU glue” and a combination of a metal home-made roller “made of matches and acorns” and my favorite soldering station as a working tool.

The algorithm of the procedure is similar to the algorithm for sealing a broken boat. First, cleaning the place of the future adhesive seam with sandpaper, degreasing (acetone perfectly prepares the PVC surface for gluing with polyurethane), then applying glue on two sides, heating with a hairdryer, folding sticky surfaces together and rolling with a roller. And so on until you get what you want. I have something like this:

or this (hermetic wallets with a transparent PVC window for a smartphone / navigator / logger)

By the way, an interesting remark for those who plan to glue their first pressure bag. I really missed the air release valve. Such things are in sales hermetic packaging with a frantic cost. I have been looking for a welded / glued valve for quite some time and to no avail. Boats cost incomprehensible money (comparable to the cost of a factory hermetic bag), there is no urine to wait at the Chinese auction. In the end, I just went to the children's department of the Central Department Store and bought inflatable arm ruffles. A penny price and 6 good valves at once.

They stuck in a thick PVC bag, although ugly, but reliable.

With this method, for some time and effort, you can provide yourself, your wife and all friends with excellent hermetic packaging. I’m even afraid to imagine how much the factory pressure bag of a similar capacity to my homemade products would cost me.
ps I glued the first bags in the form of an envelope, without a bottom. It's easier. In subsequent versions already made the bottom. In the same way as shown in the video .

About boat repair
It would be wrong if, speaking about PU, I would not mention my experience of gluing PVC and did not describe the algorithm of this process. In order not to torment the keyboard again, I just copied a couple of pieces from the water trip report.

The glue used is the cheapest shoe that can be found in our area. The national synonyms of polyurethane glue are Desmacol, Desmacol + Desmodure Hardener, Desmocol, Uranium, Bostic, 88, Black Tigrostik, Conveyor Belt Adhesive, UR600, Sar ”,“ Bonikol ”,“ Super-HH + hardener of enamel Izur-021 / Diur / Diur-extra / Izur-022 ”, Cosmofen,“ Cosmofen ”. Hardener - polyisocyanate, which will work equally well with any polyurethane raw material.
About pressure bags .
Do you like hermetic bags as I like them? It is likely that for every beginner / “one-time” water-tourist, right at that moment somewhere elder comrades say, “OK, don’t get loaded, instead of the shema take chalks from thick polyethylene for pickling cabbage”. For me it’s enough to go to the rafting once to understand what kind of rubbish this is, bags for cabbage, and why it’s better to buy even the cheapest, but still PVC pressure seal. Prices still bite, but in fact, after all, an ordinary bag is made of a PVC banner for advertising. I walked around the sporting goods stores, thought so, thought, and finally matured. After one of the events, we managed to beg the organizers for a banner (“the tables will be set to protect against acids”) and proceed with gluing. Plus, the work of the Master @ divecasper really warmed me upwith its germobaules and sealing bags. Looking at the photo - a lot of ideas appeared. For example, what are these works worth:

or

As a result, I have matured for the practical application of accumulated knowledge. Surprisingly, the first pancake did not come out lumpy. The glued bags, although they were unprepossessing and made of relatively thin PVC, passed two water trips quite confidently, moreover one in the deep off-season, along our “Pre-Karelian training” river Molchad. By the way, they still go. Their photo, unfortunately, has not been preserved. Then more experienced, I used “acetone”, “PU glue” and a combination of a metal home-made roller “made of matches and acorns” and my favorite soldering station as a working tool.

The algorithm of the procedure is similar to the algorithm for sealing a broken boat. First, cleaning the place of the future adhesive seam with sandpaper, degreasing (acetone perfectly prepares the PVC surface for gluing with polyurethane), then applying glue on two sides, heating with a hairdryer, folding sticky surfaces together and rolling with a roller. And so on until you get what you want. I have something like this:

or this (hermetic wallets with a transparent PVC window for a smartphone / navigator / logger)

By the way, an interesting remark for those who plan to glue their first pressure bag. I really missed the air release valve. Such things are in sales hermetic packaging with a frantic cost. I have been looking for a welded / glued valve for quite some time and to no avail. Boats cost incomprehensible money (comparable to the cost of a factory hermetic bag), there is no urine to wait at the Chinese auction. In the end, I just went to the children's department of the Central Department Store and bought inflatable arm ruffles. A penny price and 6 good valves at once.

They stuck in a thick PVC bag, although ugly, but reliable.

With this method, for some time and effort, you can provide yourself, your wife and all friends with excellent hermetic packaging. I’m even afraid to imagine how much the factory pressure bag of a similar capacity to my homemade products would cost me.
ps I glued the first bags in the form of an envelope, without a bottom. It's easier. In subsequent versions already made the bottom. In the same way as shown in the video .

Addition: at the request of APLe, I am attaching a list of foreign polyurethane adhesives.
Foreign commercial polyurethane adhesives
Table legend:
LSS : ultimate shear strength
TS : ultimate tensile strength
TM : tensile modulus
SG : specific gravity (density relative to water density).
Gap Filling : The maximum gap that liquid glue can fill, in mm.
Refractive Index : The indicator is of interest when gluing transparent plastics and glass.
Cure Time (sec): Initial cure time for standard weld strength.
Designations:
RT-room temperature (if there is a figure - this is the heating temperature), RF-curing by radiation
Circles indicate the mechanical properties of a particular adhesive relative to all adhesivesof this class: the black circle is the highest, the black and white circle is moderate, the white circle is weak.

LSS : ultimate shear strength
TS : ultimate tensile strength
TM : tensile modulus
SG : specific gravity (density relative to water density).
Gap Filling : The maximum gap that liquid glue can fill, in mm.
Refractive Index : The indicator is of interest when gluing transparent plastics and glass.
Cure Time (sec): Initial cure time for standard weld strength.
Designations:
RT-room temperature (if there is a figure - this is the heating temperature), RF-curing by radiation
Circles indicate the mechanical properties of a particular adhesive relative to all adhesivesof this class: the black circle is the highest, the black and white circle is moderate, the white circle is weak.

Published earlier:
Opus about His Majesty Clay. Part One -
Introductory Opus about His Majesty Clay. Part Two - Viva, Cyanoacrylate! Viva, super glue
Opusa about His Majesty Clay. Part four - silicones.
Whether the next article will depend on the Habr community , for subj .

References
WF Gum (ed.) (1992) Reaction Polymers: Polyurethanes, Epoxies, Unsaturated Polyesters, Phenolics, Special Monomers and Additives; Chemistry, Applications, Markets, Hanser, Munich and Oxford University Press, Oxford.
KN Edwards (ed.) (1981) Urethane Chemistry and Applications, ACS Symposium Series 72, Washington, DC.
J. Saunders and K. Frisch. Polyurethanes: Chemistry and Technology, Pt. 1, Interscience, New York (1963).
ME Kimball. Polyurethane adhesives: Properties and bonding procedures. Adhes. Age 24 (6), 21-24 (1981).
D. Dieterich and JN Rieck. Aqueous polyurethane systems and their possible uses. Adhes. Age 21 (2), 24–27 (1978).
PE Cranley. Polyurethane adhesives. In: Reaction Polymers, WF Gum, W. Riese and H. Ulrich (Eds.), P. 692, Oxford University Press, Hanser Publishers, New York (1992).
U. Meyer-Westhuis. Polyurethanes: Coatings, Adhesives and Sealants, pp. 20–22, Vincentz Narwork, Hanover, Germany (2003)
www.essentialchemicalindustry.org/polymers/polyurethane.html
AJ Kinloch. Adhesion and Adhesives, pp. 101-170, Chapman & Hall, London (1987). - organometallic PU primers
LD George. Composition US patent 2,277,083, assigned to Du Pont (1942) ./50.
AF Lewis, LM Zaccardo and AM Schiller. Polyurethane based adhesive systems and laminates prepared therewith. US patent 3,391,054, assigned to American Cyanamid Company (1968).
M. Huang and ER Pohl. Organofunctional silanes for sealants. In: Handbook of Sealant Technology, KL Mittal and A. Pizzi (Eds.), Pp. 27–49, CRC Press, Boca Raton, FL (2009).
KN Edwards (ed.) (1981) Urethane Chemistry and Applications, ACS Symposium Series 72, Washington, DC.
J. Saunders and K. Frisch. Polyurethanes: Chemistry and Technology, Pt. 1, Interscience, New York (1963).
ME Kimball. Polyurethane adhesives: Properties and bonding procedures. Adhes. Age 24 (6), 21-24 (1981).
D. Dieterich and JN Rieck. Aqueous polyurethane systems and their possible uses. Adhes. Age 21 (2), 24–27 (1978).
PE Cranley. Polyurethane adhesives. In: Reaction Polymers, WF Gum, W. Riese and H. Ulrich (Eds.), P. 692, Oxford University Press, Hanser Publishers, New York (1992).
U. Meyer-Westhuis. Polyurethanes: Coatings, Adhesives and Sealants, pp. 20–22, Vincentz Narwork, Hanover, Germany (2003)
www.essentialchemicalindustry.org/polymers/polyurethane.html
AJ Kinloch. Adhesion and Adhesives, pp. 101-170, Chapman & Hall, London (1987). - organometallic PU primers
LD George. Composition US patent 2,277,083, assigned to Du Pont (1942) ./50.
AF Lewis, LM Zaccardo and AM Schiller. Polyurethane based adhesive systems and laminates prepared therewith. US patent 3,391,054, assigned to American Cyanamid Company (1968).
M. Huang and ER Pohl. Organofunctional silanes for sealants. In: Handbook of Sealant Technology, KL Mittal and A. Pizzi (Eds.), Pp. 27–49, CRC Press, Boca Raton, FL (2009).


