Solar coffee: increasing the efficiency of solar cells due to caffeine

The morning starts hard, especially if you wake up at half past five. Outside the window, it rains, hiding under the umbrellas the faces of a few larks running to work, and owls returning home in measured steps. The alarm clock, being a scum by nature, continues with its inherent accuracy to ring for the third time. And for some reason, it begins to seem that he is doing this with irritation and reproach. Observing the rule of the third night watch signal from the Game of Thrones, you need to get out of bed on the third call and, like white walkers, wander towards the kitchen. Kettle, cup, sugar, coffee. That's it, the morning has officially begun.
This short essay clearly conveys the morning routine of many of us. And its main attribute is coffee, without which in the morning it is sometimes difficult to remember the presence of the brain in the cranium. The invigorating effect of coffee is the result of the psychostimulating effect of caffeine. What am I talking about? A group of scientists, for fun, decided to use caffeine to improve photocells. And as we know, in every joke there is some truth, because this funny idea gave amazing results in practice. How was caffeine implemented in photocells, what indicators turned out to be improved, and how much is such an improvement justified? We will find answers to these and other questions (no, not in the coffee grounds) in a report by scientists. Go.
Study basis
As I mentioned earlier, this study really originated as a joke over a cup of morning coffee in the laboratory cafeteria. However, scientists would not be scientists if they had not tried to implement something like that, albeit ridiculous at first glance.
In addition to caffeine, the main experimental one was not a simple photocell, but perovskite.
Photocell * is an electronic device for converting photon energy (sunlight) into electrical energy.
Perovskite * is a rare mineral of calcium titanate (CaTiO 3 ).The perovskite photocell is based on materials from an organic-inorganic hybrid of perovskite halide (hereinafter PVSK ). PVSK is a real breakthrough in solar energy, which is confirmed by usage statistics: 3.8% in 2009 and 23.3% at the end of 2018. However, rejoice at the success of this material so far only in laboratory conditions, because problems with long-term stability do not allow its use in the commercial production of solar cells. For example, PVSKs based on cesium (Cs) and formamidinium (FA), which are popular in research, cannot work normally at room temperature in terms of thermodynamic properties. But it can methylammonium (PV) based PVSK.
But this is not so simple either: the MA PVSK organic cation is volatile, which results in the rapid decomposition of PVSK and precipitation of trigonal lead iodide (PbI 2 ) at elevated temperatures.
There is also a problem with the ions inside the PVSK. Researchers give a vivid example: I - ion can easily pass through polycrystalline grains of PVSK and go beyond the PVSK layer, and then act on a metal electrode under the influence of thermal energy. This causes defects in the form of sections of non-radiative recombination. In addition, randomly oriented PVSK grains can lead to poor charge transfer in the vertical direction, which is a consequence of the fast and uncontrolled growth process of the PVSK film.
According to scientists, the overwhelming majority of work on improving the performance of PVSK-based solar cells was aimed at the devices themselves, their architecture and structural improvements, rather than PVSK.
In this same study, scientists applied 1,3,7-trimethyl-xanthine, a fanciful scientific name for caffeine (Lewis structure and 1A three-dimensional model below) to PVSK based on methyl ammonium (MA ). Using carboxyl groups under various chemical conditions, caffeine became a kind of “molecular gate” that interacted with Pb 2+ ions , slowing the growth of PVSK crystals. In addition, it was possible to achieve the desired orientation by increasing the activation energy.
As a result, it was possible to achieve excellent crystallinity of caffeinated PVSK films and lower defect density, as well as better vertical charge transfer. And the obtained coefficient of performance (COP) was previously unthinkable for this technology 20.25%. As for the thermal stability of the device, the scientists managed to achieve stability at a temperature of 85 ° C for more than 1300 hours.
These are really excellent results, especially considering the comic roots of this study. Now let's take a closer look at what and how it worked.
Research results
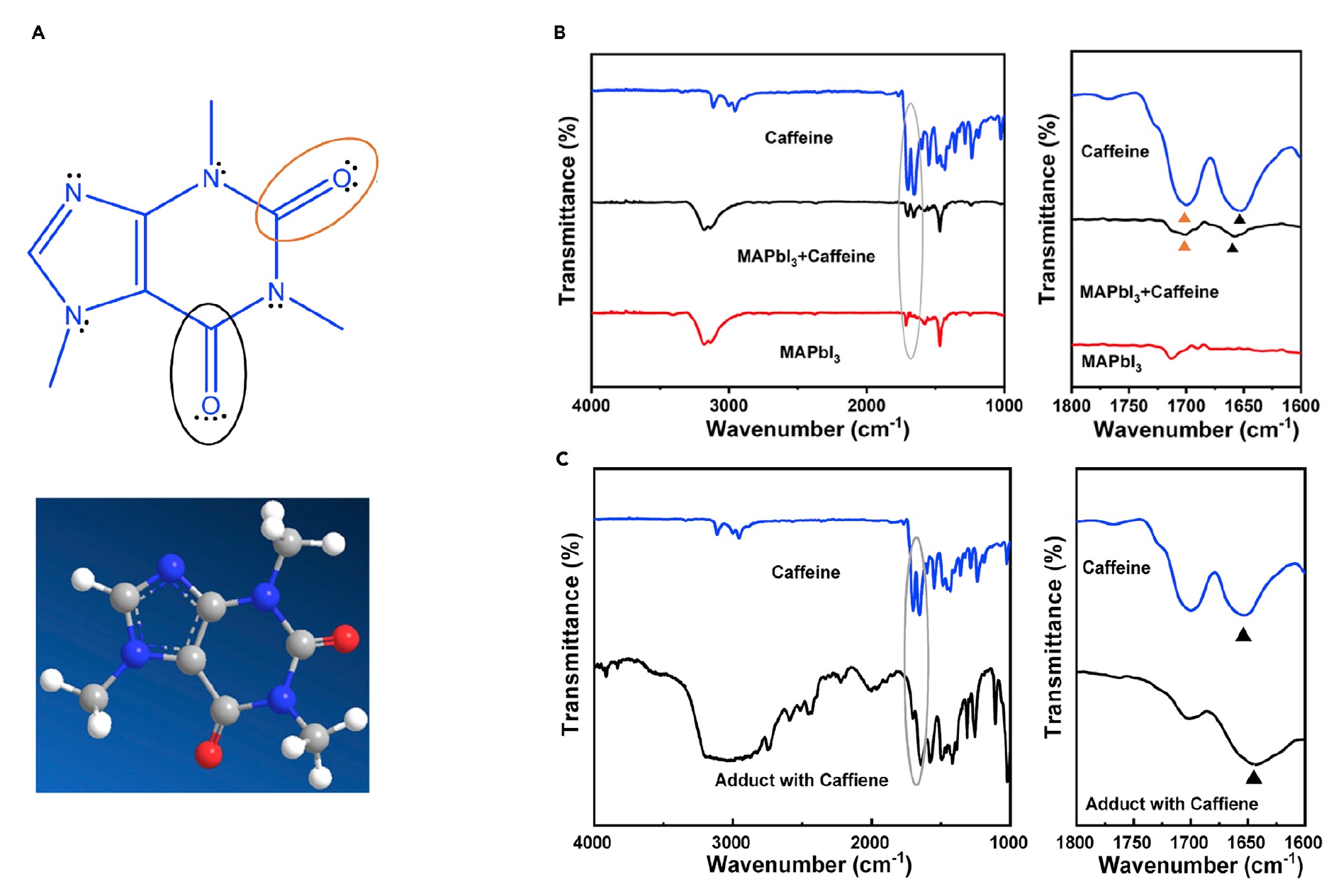
Image No. 1
Image 1B shows the results of infrared spectroscopy with Fourier transform of caffeine (blue line), pure MAPbI 3 (black line) and MAPbI 3 with caffeine (red line). The stretching vibrations related to the two C = O bonds in pure caffeine appear at 1.652 cm -1 and 1,699 cm -1 . When caffeine was added to the MAPbI 3 film , a stretching shift of C = O was observed with a lower frequency from 1.652 to 1.657 cm -1 , while the vibrational mode C = O by 1.699 cm -1 retains its original value. This is an indication that caffeine is present in the MAPbI 3 film.after annealing, and possibly formed an adduct with MAPbI 3 through the interaction between Pb 2+ in PVSK and one of the C = O bonds in caffeine.
To further confirm the effect of caffeine on PVSK, scientists conducted a PbI 2 -MAI-DMSO-caffeine adduct spectroscopy , which also showed a C = O stretch shift from 1652 to 1643 cm -1 ( 1C ).
These observations confirm that the interaction between C = O in caffeine and Pb 2+ ionsforms a molecular gate, increasing the activation energy. And this, in turn, slows down the PVSK crystal growth process, improving the overall crystallinity of PVSK films. In addition, this molecular gate may interact with amorphized PVSK upon heating, which can prevent thermal decomposition.
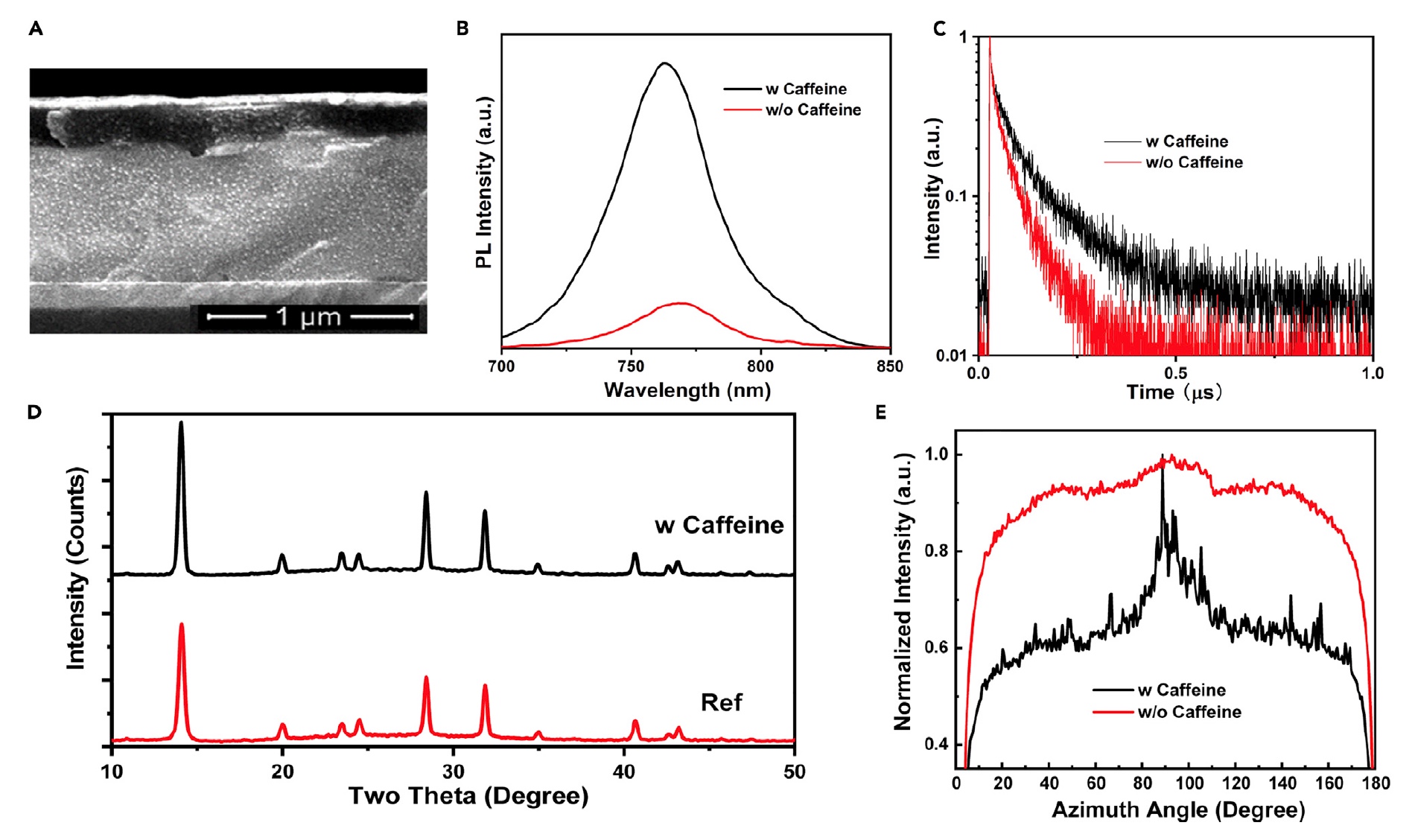
Image 2
Image 2A is a SEM image of a cross-section of a caffeinated PVSK film. Changes in the attenuation of stationary photoluminescence ( 2B ) and temporal resolution photoluminescence ( 2C) were carried out to study the quality of the film and the dynamics of charge recombination. The photoluminescence intensity of a caffeinated PVSK film (black lines) was 6 times higher than that of caffeine-free films (red lines). A blue shift from 770 to 763 nm was also noted, which once again confirms the decrease in the number of defects during the incorporation of caffeine into the structure of the PVSK film.
Next, an X-ray diffraction analysis was performed to study the crystal structure of the PVSK film deposited on a substrate of indium tin oxide ( 2D ). And for films with and without caffeine, no diffraction peak was found at 12.5, which corresponds to the (001) planes of hexagonal PbI 2. Both films showed the same tetragonal PVSK phase with a dominant (110) reflection of the lattice at 13.9, which is an excellent orientation for the studied PVSK films. The ratio of peak intensity (110) at 13.9 to peak intensity (222) at 31.8 increased from 2.00 to 2.43 when caffeine was added. This indicates a faster growth of (110) grains that absorb randomly oriented grains.
Grain sizes were also measured using the Scherrer formula and the half-width of the (110) peak. With the introduction of caffeine, the grain size increased from 37.97 to 55.99 nm.
Figure 2E shows us a graph of the normalized azimuthal angle along the (110) plane of MAPbI 3 filmscaffeine-free (red line) and caffeine (black line). At an angle of 90 °, a caffeinated film shows a fairly pronounced peak compared to a decaffeinated captive. A narrower half-width suggests that caffeine contributed to the growth of PVSK grains along the plane, which improves charge transfer.
Next, scientists conducted an analysis of transient photocurrent ( TPC ) and transient photovoltaic voltage ( TPV ).
The experimental photocells were manufactured taking into account the nip planar structure, and indium tin oxide (ITO) acted as an anode. In turn, tin oxide nanoparticles were used as an electron transport layer. Both pure MAPbI 3 and caffeine-containing MAPbI 3 acted as an active layer.. The role of the hole transport layer (quasiparticles with positive charge) was played by poly [bis (4-phenyl) (2,4,6-trimethylphenyl) amine] ([C 6 H 4 N (C 6 H 2 (CH 3 ) 3 ) C 6 H 4 ] n ) doped with 4-isopropyl-40-methyldiphenyl-iodonium tetrakis (pentafluorophenyl) borate (C 40 H 18 BF 20 I). Silver (Ag) was used for the cathode.
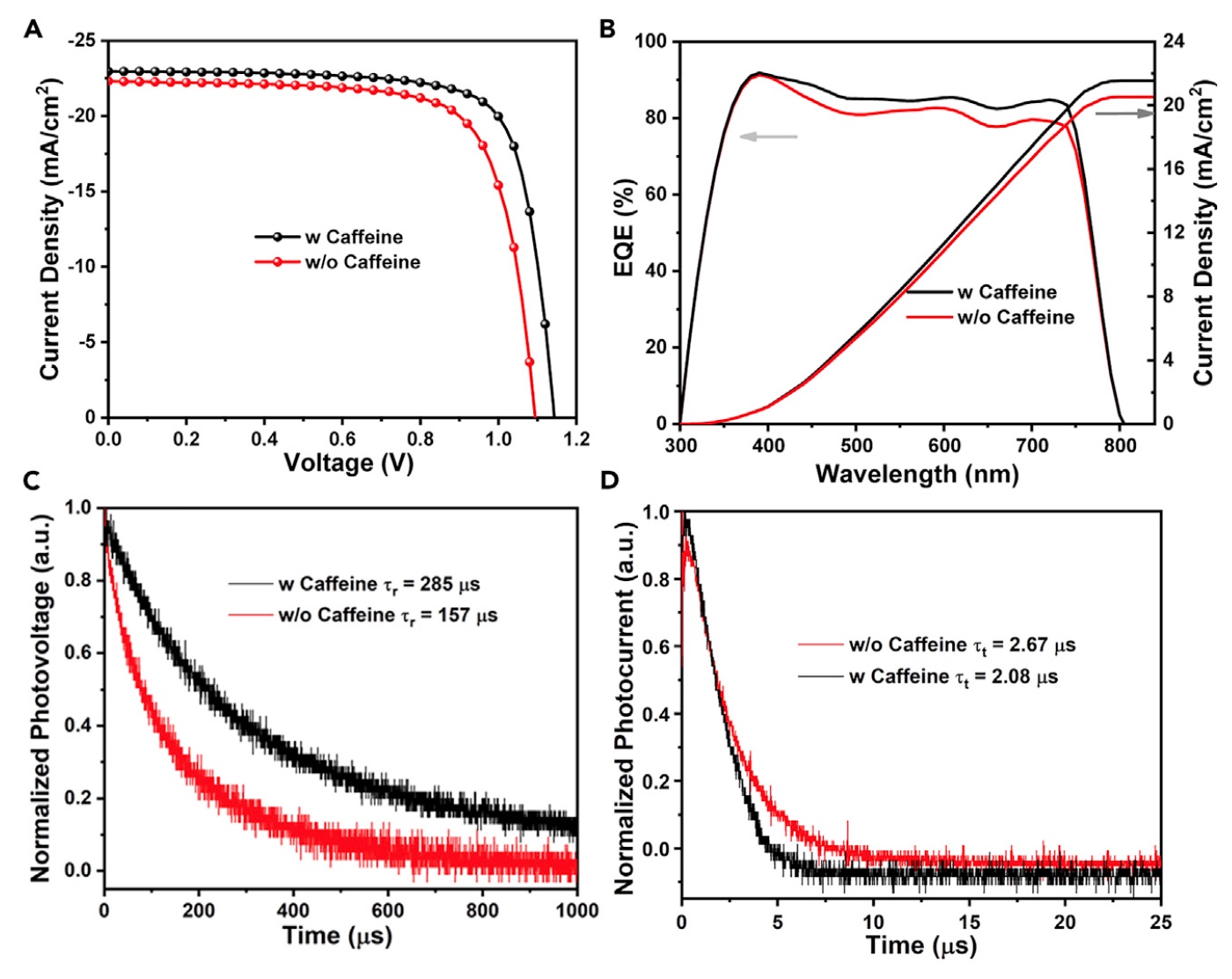
Image No. 3
Image 3A shows JV curves (current density, mA / cm 2 ) of devices based on pure MAPbI 3 and MAPbI 3/ caffeine obtained using artificial sun AM1.5G with an intensity of 100 mW / cm 2 . The percentage of caffeine incorporated into the system ranged from 0 to 2% of the total mass.
An increase in the amount of caffeine introduced to 1% led to an increase in some characteristics, namely: open circuit voltage (V oc ), short circuit current (J sc ), duty cycle (FF), and reproducibility.
The maximum efficiency (PCE in the table below) of pure (caffeine-free) MAPbI 3 was 17.59% (V oc : 1.074 V, J sc : 22.29 mA / cm 2 , FF: 73.46%). But if there is 1% caffeine in the system, the efficiency indicator increased to 20.25% (V oc: 1.143 V, J sc : 22.97 mA / cm 2 , FF: 77.13%). Scientists attribute the
increase in V oc and FF to a decrease in non-radiative recombination and crystalline defects, which is a result of passivation due to the incorporation of caffeine into the system. The parameter J Sc also increased from 22.29 to 22.97 mA / cm 2 (graph 3B ).
For a more detailed study of the effect of caffeine on system performance, scientists conducted a comparative analysis of the kinetics of charge transfer and charge recombination of solar cells with and without caffeine. The analysis showed ( 3C ) that the lifetime of charge recombination (t r) devices with caffeine (285 ms) were significantly longer than decaffeinated (157 ms). It follows from this that the concentration of defects is much lower. In this case, the charge transfer time (t t ) when caffeine was added to the device decreased from 2.67 to 2.08 ms.
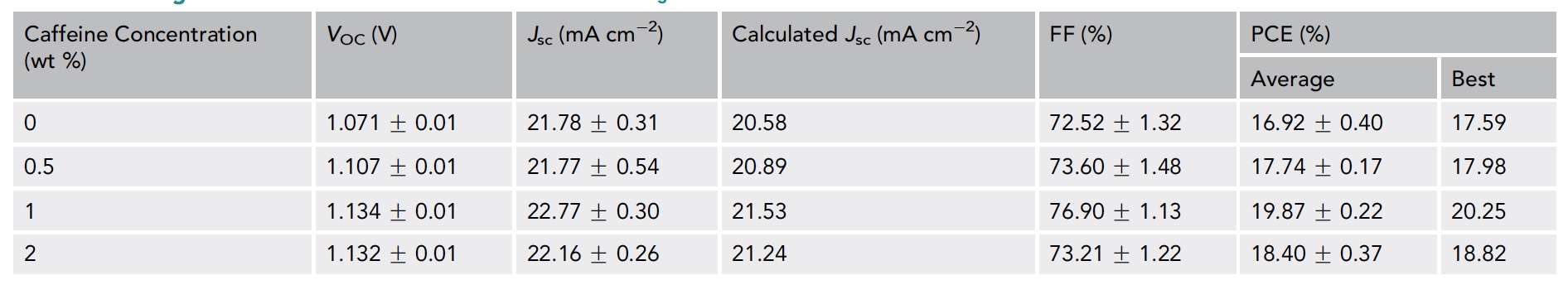
Table of indicators depending on the concentration of caffeine.
In order to confirm the effect of molecular shutter of caffeine in photocells during the process of thermal decomposition, scientists conducted a test for resistance to constant thermal loads: 85 ° C in a nitrogen medium.
The caffeinated device showed excellent thermal stability, retaining 86% of the original efficiency after 1300 hours. But the decaffeinated device under the same conditions retained only 60% of the primary efficiency. Scientists attribute this to ion migration, poor crystallization, and phase instability of pure MAPbI 3 at high temperatures.
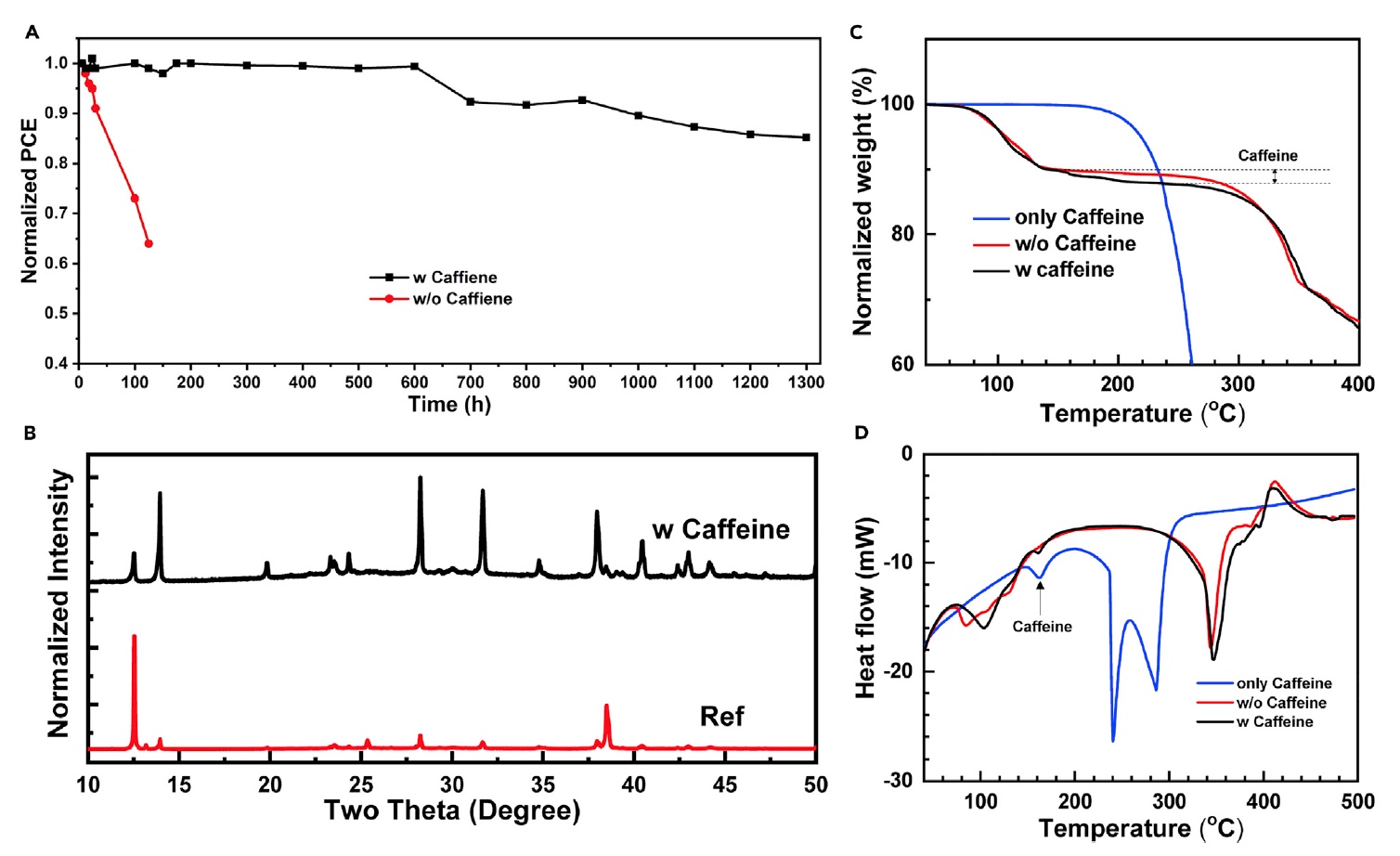
Image No. 4
Scientists needed to understand in more detail the effect of caffeine on the functioning of solar cells in terms of ion migration and phase decomposition. To do this, an X-ray diffraction analysis ( 4B ) of the devices was carried out after thermal stability tests.
The caffeine-free device showed a fairly large peak at 12.5, associated with the (001) plane of hexagonal PbI 2. Very weak diffraction at 13.9 implies complete degradation of the PVSK crystal. But relatively strong diffraction of 38.5 was observed relative to the (003) plane of PbI 2 .
As mentioned earlier, the very good crystallinity of PVSK due to the addition of caffeine should prevent the migration of ions during heating. Thermogravimetric analysis of caffeine and adduct was carried out to establish the phase stability and thermal properties of caffeine and the intermediate phase of the adduct. Graphs 4C and 4D show mass loss and heat flux of caffeine, pure PVSK and PVSK + caffeine.
The analysis showed that caffeine completely decomposes at a temperature of about 285 ° C, while it showed excellent thermal stability at temperatures below 200 ° C. On graph 4C we can see three stages of mass loss of pure PVSK: 70 ° C, 340 ° C and 460 ° C. This is due to the sublimation of DMSO, MAI, and PbI 2 , respectively. The sublimation temperature of MAI and PbI 2 for PVSK + caffeine was significantly higher, indicating the need for more energy to break the bond between caffeine and PVSK. This statement is supported by an analysis of heat fluxes ( 4D ). Thus, the connection between caffeine and PVSK forms a molecular gate, which increases the rate of necessary decay activation energy during heating.
For a more detailed acquaintance with the nuances of the study, I strongly recommend that you look into the report of scientists and additional materials to it.
Epilogue
This study showed that the introduction of caffeine in PVSK materials allows to obtain photoelectric cells with high efficiency, reduce ion migration, reduce the number of defects and enhance thermal stability. The use of PVSK materials began not so long ago, but it is already considered the most promising branch of solar energy. And this means that it is necessary to improve all aspects of this technology if we want to get devices that will have high performance at low cost. This work just relates to research aimed precisely at this.
Using caffeine in the development of solar cells sounds like a joke, it was a joke over a cup of coffee in the morning in the laboratory. But jokes are bad with scientists, and any, even the strangest idea, can give an excellent result if you apply knowledge, ingenuity and a little creative approach.
Thank you for your attention, remain curious and have a good working week, guys.
Thank you for staying with us. Do you like our articles? Want to see more interesting materials? Support us by placing an order or recommending it to your friends, a 30% discount for Habr users on a unique analogue of entry-level servers that we invented for you: The whole truth about VPS (KVM) E5-2650 v4 (6 Cores) 10GB DDR4 240GB SSD 1Gbps from $ 20 or how to divide the server?(options are available with RAID1 and RAID10, up to 24 cores and up to 40GB DDR4).
VPS (KVM) E5-2650 v4 (6 Cores) 10GB DDR4 240GB SSD 1Gbps until the summer for free when paying for a period of six months, you can order here .
Dell R730xd 2 times cheaper? Only we have 2 x Intel TetraDeca-Core Xeon 2x E5-2697v3 2.6GHz 14C 64GB DDR4 4x960GB SSD 1Gbps 100 TV from $ 199 in the Netherlands! Dell R420 - 2x E5-2430 2.2Ghz 6C 128GB DDR3 2x960GB SSD 1Gbps 100TB - from $ 99! Read about How to Build Infrastructure Bldg. class c using Dell R730xd E5-2650 v4 servers costing 9,000 euros for a penny?
