Ask Ethan # 34: How the Universe Uses Its Fuel
- Transfer
If people could be measured by the periodic table of elements, then love would be under the first number.
- David Mitchell
The reader asks:
What do scientists know about how much hydrogen was originally created in the universe, and what happened to it? I would like to know how much it is in stars, how much has turned into heavier elements, how much it is in planets, moons, comets, in interstellar space, intergalactic, and somewhere else where I forgot.
You can start only from the very beginning - from the moment of the formation of the Universe visible to us, that is, from the Big Bang!

At the end of cosmic inflation and after energy was transformed into matter, antimatter and radiation, what appeared to be what we call the "visible part of the Universe." Initially, it was filled with a hot and dense soup of ultra-relativistic particles, and then it began to cool and expand - and the expansion rate decreased significantly over time. Matter defeated antimatter, residues annihilated, quarks and gluons formed protons and neutrons - and all this happened in a sea of abundant radiation, which prevailed over all protons and neutrons.
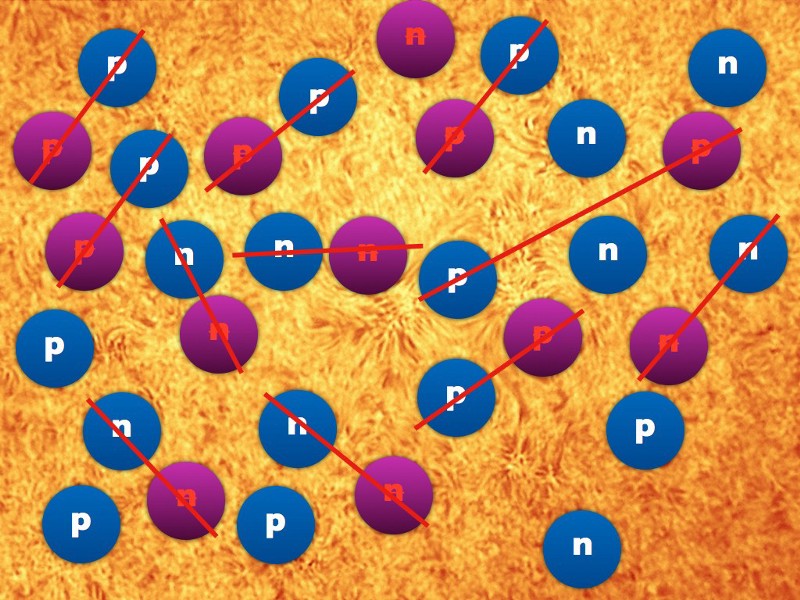
After a second from the moment of the Big Bang, that part of the Universe that we can observe today contained 10 90radiation particles, 10 80 protons and neutrons (so far in a ratio of about 50/50). Most neutrons either turned into protons, catching a neutrino or decaying, and after three minutes the remaining neutrons gathered together with the protons and formed helium.
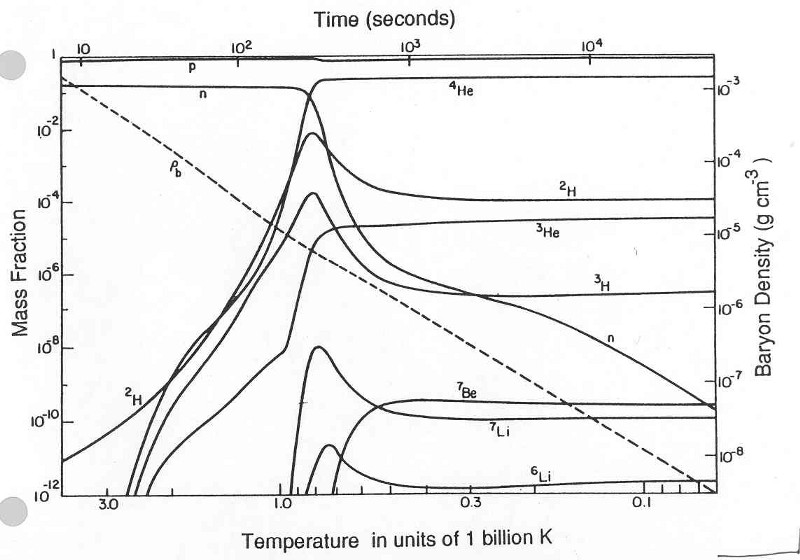
By the age of 8 minutes, 92% of all atomic nuclei (in quantity) were hydrogen atoms, and 8% - helium. Since helium is 4 times heavier, by weight this ratio looked like 75/25.
Over time, the Universe cooled down, formed neutral atoms after several hundred thousand years, and after millions of years these atoms cooled and gathered in giant clouds of molecular gas. And, despite the fact that in those days, electromagnetic interaction and gravity had unusual properties, a nuclear reaction is required to change the type of atom. Therefore, from the point of view of hydrogen, little has changed during this time. Until the stars appeared.

When you create a star, in its core the light nuclei of atoms begin to turn into heavier ones. The process of nuclear fusion occurs at huge temperatures, pressures and densities - when a mass of hydrogen of at least tens of thousands of Earth masses is compressed into one dense structure. When the temperature exceeds four million Kelvin, the synthesis begins. The first stage of synthesis is protons, i.e. hydrogen nuclei scramble up the nuclear ladder, forming helium.
How fast does hydrogen run out? The determining factor here is the mass of the star.

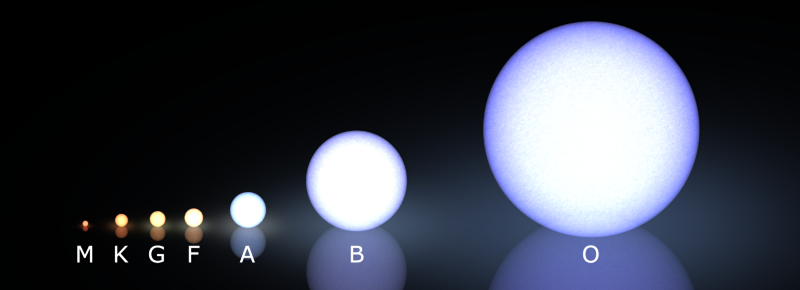
In superheavy stars, hundreds of times the mass of the sun, the nuclei burn hydrogen very quickly - in just a few million years. Such O-class stars are very rare, there are only 0.1% of the total - but they are the brightest stars in the entire Universe.
The lightest stars of the M-class, from the main sequence, are too dim for even Hubble to fix. They live for tens and hundreds of trillions of years (more than 1000 more than the current age of the Universe) before they spend all of their hydrogen. Moreover, such M-class stars are the most common in the Universe; these are approximately three out of every four stars.
One could decide that after all generations of stars that lived and died over 13.82 billion years, and taking into account the huge number of elements heavier than hydrogen on Earth and in the solar system, much less hydrogen could be found in the Universe today.
But this is not so.

Our Sun was formed when the Universe was 9 billion years old, in the plane of a spiral galaxy - one of the most enriched places in the Universe. But at the same time, having formed, it consisted (by mass) of 71% hydrogen, 27% helium, and 2% of everything else. If we count everything into atoms and take the Sun as the standard, we will find that the amount of hydrogen in 9.3 billion years of the life of the Universe has decreased from 92% to 91.1%.
Just. How did it happen?

When a molecular cloud is compressed, only from 5% to 10% of the mass of the cloud falls into the star. The rest is emitted into the interstellar space by ultraviolet radiation emitted by new stars.

In addition, all stars heavier than the M-class burn only 10% of all fuel before turning into a red giant. For small-mass stars, combustion is slow enough to complete convection, when spent "fuel" moves from the core to the outer layers, and unburned hydrogen moves inward. A star like Proxima Centauri will eventually turn 100% of its hydrogen into helium - and it will take several trillion years.
But all heavy stars will burn up to 10% of the fuel, and die as supernovae or as planetary nebulae, and return the bulk of hydrogen back to interstellar space.
Of course, associations of galaxies constantly occur, during which periods of intense birth of stars, known as star formation, occur.

But the more actively star formation occurs, the more hydrogen is ejected away from the galaxy, into intergalactic space. And today, about 50% of the hydrogen in the Universe does not belong to any galaxy, but occupies the space between them. Most likely, stars will never form from it. In addition, the rate of star formation has drastically decreased over time - now it is only 3% of the maximum that was once.

Galaxies remain bound structures in which a large amount of hydrogen will be contained. And although, most likely, stars will no longer form there in the way that prevails now, we believe that new stars will appear even trillions of years, and maybe even longer.
The universe will darken, but not because it runs out of hydrogen. This will be because the remaining hydrogen will not be able to collect in molecular clouds large enough to form stars. According to estimates, there is every reason to believe that its amount in the Universe will not fall below 80%. That is, we will have a lot of helium and many even heavier elements, but even with time tending to infinity, the Universe will be composed mainly of hydrogen.
Its mass can fall below 50%, mainly due to large galaxies and their clusters. But we believe that when the age of the Universe is a million times greater than now, new stars will form, but according to completely different schemes - due to the compression of molecular clouds weighing millions of times more than the Sun.

Will this process come to an end? It is not possible to calculate this, and the Universe is still too young to be able to draw such conclusions from observations.
But, as far as we know, hydrogen was originally the most widespread element in the Universe, and it will remain in this position as long as there is a Universe in which it can exist.
