Chasing the blue LED

Recently, a lot of informative articles about LED lamps , their circuitry and production have appeared on Habré and Giktayms . The development of their main component - the blue LED - took a quarter of a century, and the authors of the most successful technologies were awarded the Nobel Prize this fall . I would like to highlight this story from the side of physics and tell why the path to the blue diodes was so long and thorny.
Introduction
On Habré already in detail talked about the basics of semiconductor electronics and about how the LED works . Briefly recall the main points. If a direct voltage is applied to the pn junction, then electrons from the n-region and holes from the p-region will move towards each other and recombine, emitting energy in the form of photons.

Source
Now let's look at the zone diagram. The applied voltage casts electrons into the conduction band (respectively, holes into the valence band). Upon meeting, they recombine.

A source
It can be seen that the energy of the emitted photons is approximately equal to the band gap. Actually, this determines the wavelength and color of the radiation. So, the energy of quanta of blue light is greater than red - therefore, for a blue LED, a semiconductor with a larger band gap is needed. Historically, such semiconductors are called wide-gap .
Generally speaking, there are not so many semiconductors in the world, and their basic properties are well understood. This graph is very informative (Zhores Alferov in his Nobel lecture calls it the “world map” of semiconductors):
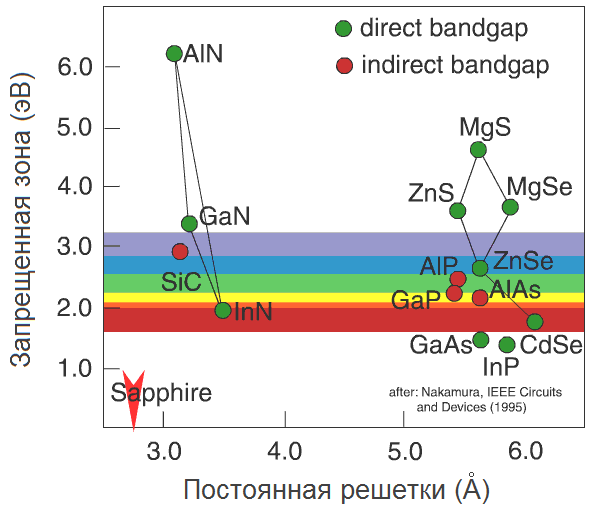
The lattice constant is laid out horizontally hereone or another semiconductor - roughly speaking, the distance between two neighboring atoms in a crystal (we will return to it later). Vertical - band gap in electron volts (eV). To have an idea, a person sees photons with energies from 1.8 eV (wavelength 700 nm, red) to 3.1 eV (400 nm, violet). We are interested in the blue-violet region, with a margin of about 2.6–3.3 eV.
Lyrical digression: electronvolts
According to the definition, an energy of 1 eV is enough to reduce the electron potential by 1 Volt. And vice versa: if we increase the electron potential by 1 Volt (say, after it runs from “-” to “+” of a single-volt battery), then it will receive an energy of 1 eV. Thus, if the semiconductor band gap is 3 eV (violet LEDs), then to throw an electron into the conduction band, you can increase its potential by 3 V. In general, this determines the operating voltage on the LED, reaching as much as 3–3.5 V for blue / violet diodes.
As we see, only three semiconductors fall into the blue-violet region: SiC, ZnSe, and GaN. Historically, in this order, they entered the "LED" arena.
1. Silicon Carbide (SiC)
Silicon carbide is remarkable in that it is able to form a huge number of crystalline modifications. Already in the 50s, this made it possible to create structures with different bandgaps - and therefore, generate radiation in different parts of the visible spectrum. After the red and yellow LEDs, the first blue LED was developed in 1969. In the 80s, they became available commercially.
However, with all the technological successes, the efficiency of the devices did not exceed 0.03%. The reason was fundamental. To understand it, we have to go a little deeper into physics.
Direct-gap and indirect-gap semiconductors
In fact, the band structure of a semiconductor looks a little more complicated than in the figure above. The position of the bands (and the band gap) depends on the momentum of the electron in the crystal (i.e., actually on its speed). Moreover, it depends on the vector: not only on the magnitude, but also on the direction. The result is something like this:
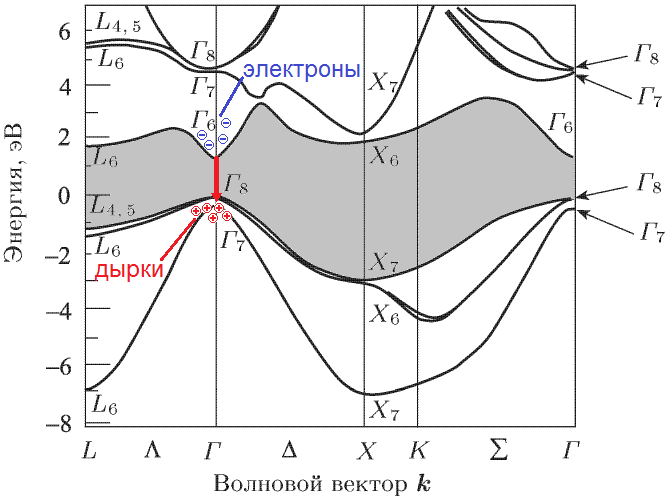
Source: [1]
This is the band structure of gallium arsenide. Vertical energy is traditionally set aside. Horizontal - without going into details - the different possible values of the vector momentum (which is also called the wave vector or electron position in the Brillouin zone) are shown) For example, the electrons in the gamma point (any of the letters G in the picture) have a zero momentum. For clarity, I painted gray. Now we look at the red arrow: the electron from the lower point of the conduction band falls to the upper point of the valence band without changing the momentum (the same horizontal coordinate), emitting a photon. This is a direct transition.
But what happens in SiC?
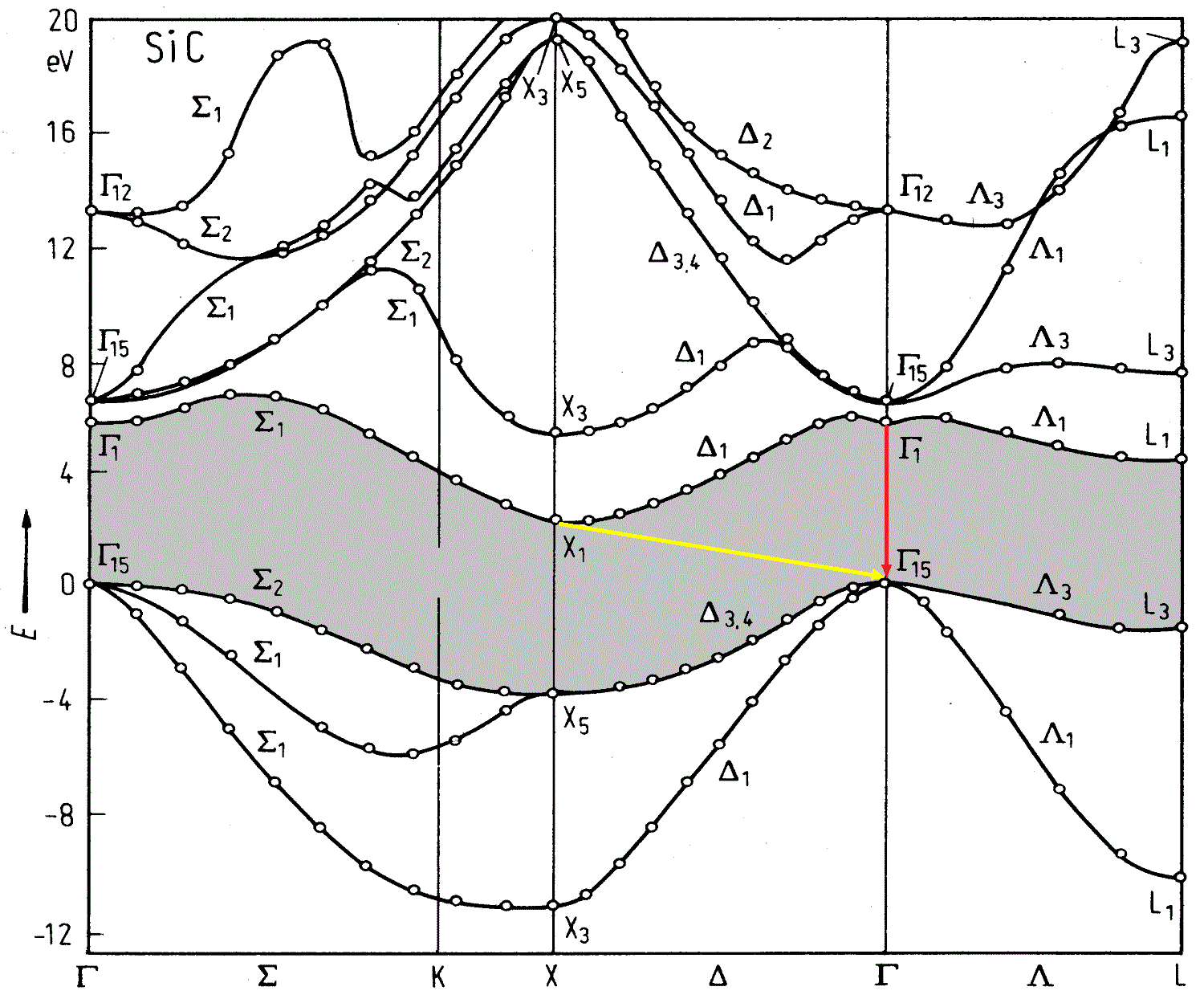
Source
We look at the yellow arrow. Electrons from the bottom point do not fall vertically down, but at an angle ( indirect transition) - which means they change their momentum. The problem is that for a radiated photon this pulse is too large, and no one canceled the law of conservation of momentum. Need another particle - phonon- able to compensate for the electron momentum. True, now our process from two-particle (electron - photon) has become three-particle, which significantly reduced its probability. For an LED, this means that of all the electrons in the conduction band, only a few can emit a photon - which means we will noticeably lose in efficiency.
Question: but there’s a red arrow in the picture, is everything okay with her?
Answer: yes, the transition is direct, but its energy is almost 6 eV (good ultraviolet, you can burn your eyes). For us it’s too much.
Actually, it was the indirect SiC transition that predetermined its fate (or rather, its end) in optoelectronics. Fortunately, ZnSe and GaN turned out to be direct-gap semiconductors.
2. Zinc Selenide (ZnSe)
ZnSe differed from SiC not only in
Firstly, there was a problem with the manufacture of contacts to the diode. The contacts must be metallic, and a Schottky barrier will inevitably form at the metal-semiconductor interface . The trouble with ZnSe was that there was a huge voltage drop on the Schottky barrier. As a result, the operating voltage of the diode reached 10 V instead of the expected 2.8. Later it was possible to reduce it to 4.2 V, and then only at the cost of tangible complication of production.
The next problem was the heat sink. The low electron mobility in ZnSe leads to high active resistance (and, as a consequence, high heat release); low thermal conductivity makes the situation even worse.
Finally, the most serious obstacle was the degradation of the material in the process. The essence of this process is reduced to a sharp increase in the number of defects in the crystal at high current or radiation densities. This wildly reduced the lifetime of the device (in general, it is difficult to talk about the “lifetime” when the laser diode burns out after 20 seconds of operation).
Thus, only low-power blue diodes became the lot of zinc selenide. There was one more material left in the reserve, the possibility of which at first few believed.
3. Gallium nitride (GaN) and other III-nitrides
To understand why GaN diodes returned only in the mid-80s, you need to remember how LEDs are produced in general. Thin layers of semiconductors (in fact, all electronics are more complex than a transistor) are grown by epitaxial methods. The essence of this word lies in the fact that we deposit atoms on the surface of a semiconductor that do not lie separately, but in a very orderly manner - repeating and, as it were, continuing up the crystal lattice. (This is simply energetically beneficial.) As a result, layer by layer we get an almost perfect crystal.

Source The
subtlety here is that we cannot start epitaxy from scratch. We need an initial single crystal - a substrate .
Lyrical digression: substrate growth
Probably many of the readers of Giktayms grew salt crystals in childhood. It was necessary to tie a seed with a thread - a grain of salt - and hang it in a saline solution. So, single crystals of semiconductors grow in about the same way - of course, from components of the highest purity, while still rotating the seed and slowly pulling it up. This is called the Czochralski method . The grown cylinder is cut into thin plates - future substrates, then they are polished, packaged in special clean containers and sent to production.

Sources: 1 , 2 .

Sources: 1 , 2 .
The rest is simple: we take a silicon carbide substrate, and we grow an silicon carbide diode on it with epitaxy. Although there will be a problem with ZnSe: substrates from ZnSe are not made so widely. Then let's take the GaAs substrate: judging by the “world map”, the lattice constants of GaAs and ZnSe are almost the same (5.66 Å), which means growth will go well. The problem would be if we took a silicon carbide substrate with a lattice constant of about 3 Å: ZnSe could not repeat such a close lattice due to internal stresses (in fact, repulsion between Zn and Se atoms). Even on silicon (5.4 Å, the difference is only 5%) it would be problematic to grow ZnSe - starting from a certain layer, the internal stress in the ZnSe layer would become too great, and he would want to grow from the usual lattice constant. The transition from one grid to another would look something like this:

Source
That is, the mismatch of the gratings leads to the appearance of such defects. And defects in optoelectronics are very bad. Moral: choose the right substrate for epitaxy!
Solid solutions
Take a ZnSe crystal and replace all the zinc atoms with magnesium atoms. The result is an MgSe crystal, which has slightly different lattice constant (the magnesium atom is larger than the zinc atom) and the band gap. But what if not all atoms are replaced, but only some? Say five percent? Something in between will turn out: the shape of the crystal lattice will remain the same, but the lattice constant and the band gap will be somewhere between them for ZnSe and MgSe. Such a substance is called a solid solution of ZnSe and MgSe, denoted as Zn 0.95 Mg 0.05 Se and on the “world map” lies on a straight line connecting ZnSe and MgSe.

Actually, the straight lines on the “world map” show for which pairs of semiconductors stable solid solutions exist. Thus, by controlling the concentrations of the components during growth, we can adjust both the lattice constant and the band gap of the semiconductor - and therefore, control the radiation wavelength. Thus, the addition of indium to GaN allows one to switch from ultraviolet (3.4 eV) to the visible blue region (2.7–2.9 eV).
Substrate for GaN
Now it’s clear what the value of the “world map” is: it allows you to select solid solutions to control the wavelength and the corresponding materials for the substrate. Well, let's pick up a substrate for InGaN growth (it so happened that GaN substrates cannot be grown). Wait, how is it - there are no suitable substrates? Not at all?

SiC is close, but not optimal (the difference in constant lattices is 4%) and also expensive. Because of the price, sapphire was mainly used.
Actually, the lack of substrates was the reason why everyone worked with zinc selenide in the 80s. It was believed that the quality of growth should be a major success factor. Almost the only exception was the Isamu Akasaki group at Nagoya University. By 1986, they had proposed a tricky solution to the problem. The bottom line was that several intermediate (so-called buffer ) GaN layers were grown on a sapphire substrate . The concentration of defects in them was huge, but decreased from layer to layer. Later, Shuji Nakamura (Nichia Corp.) improved this process using AlGaN buffers. The structure was as follows:

Source
In an electron microscope, it looked something like this:

On the left is GaN on sapphire, on the right to compare ZnSe on GaAs (the GaAs substrate is not shown, but judging by the size it is not far). Pay attention to the scale: GaN is one and a half times thicker. Sources: 1 , 2 .
So, even after all the improvements, the dislocation density remained huge. And defects for optoelectronics are not just bad, but very bad: they capture media very well and allow them to recombine non-radiation, dramatically reducing efficiency. This is the difference between the diode and the LED: making a pn junction is not a problem, making a defective pn junction is a difficult technological task. For example, a GaAs LED with a concentration of defects, as in the picture, would obviously not work (more precisely, it would, but in the best case, just like a diode).
Nature intervened here: it turned out that the resulting quality is quite enough for the normal operation of the LEDs. The reasons for this are extremely nontrivial and not fully understood. One of them is not the usual mobility of carriers in GaN: they do not travel long distances, and therefore can avoid meeting with a dislocation. Moreover, almost all dislocations in GaN are filiform, extending from the substrate to the surface of the diode. This imposes some restrictions on their properties, which can be used in practice.
Incredible, but true: the biggest gallium nitride problem suddenly ended up behind.
Acceptors in GaN
Another challenge was the p-doping of gallium nitride. In the early stages , magnesium was used as an acceptor (material for p-doping). However, the resulting material did not behave like a p-type semiconductor. There was some unknown factor that prevented magnesium from playing the role of an acceptor.
The easiest way to determine the degree of alloying is to measure the resistance of the material. In p- and n-doped semiconductors, it is low due to an excess of carriers (electrons in n- or holes in p-type). In a pure semiconductor, there are no free carriers, therefore, its resistance is high.
The following episode is noteworthy. Akasaki was able to show that irradiation of GaN with a low-energy electron beam can solve the problem: magnesium began to behave as an acceptor. True, in the production process, the material again ceased to be conductive. This happened during annealing : the structure was kept at high temperature in ammonia. This is a standard procedure for reducing stresses in the crystal lattice.
Nakamura noted that p-conductivity disappears at an annealing temperature above 400 ° C. At the same temperature, the dissociation of ammonia to nitrogen and hydrogen begins. But what if the problem is somehow related to hydrogen? Detailed studies showed that Nakamura was right: atomic hydrogen penetrated the structure, bound to magnesium and shared his electron with it, preventing it from playing the role of an acceptor. Annealing in nitrogen instead of ammonia immediately solved the problem:

Upper curve: when annealing in ammonia above 400 ° C, the resistance of the material increases sharply. In nitrogen (lower line) this does not happen. A source.
Later it turned out that annealing in nitrogen does not just solve the problem with ammonia - it is able to completely replace the electron beam. That is, instead of what Akasaki did, you can simply anneal the material in nitrogen: this will break the bonds of magnesium with hydrogen and make the material p-conductive. Technologically, this was extremely important: processing large crystals with an electron beam is not a quick and expensive business.
Happy end

Shuji Nakamura. A source.
The story did not end there, but the main problems were resolved. Along the way, I managed to do a lot of interesting things, for example, to develop a special reactor for the growth of III-nitrides. By 1994, Nichia Corp. started the industrial production of blue InGaN LEDs.
Sources
[1] P. Yu., M. Cardona “Fundamentals of the physics of semiconductors” - M .: Fizmatlit, 2002. - 560 p.
[2] S. Nakamura, S. Pearton, G. Fasol “The Blue Laser Diode: The Complete Story” - Springer, 2000. - 368 p.
[3] H. Morkoç et al. J. Appl. Phys. 76, 1363 (1994).
[4] http://www.eurotechnology.com/store/solidstatelighting/
[5] http://www.nobelprize.org/nobel_prizes/physics/laureates/2014/ (title picture also from here)
UPD. Thanks to Mercury13 and jar_ohty for comments on ammonia.
