Diagnosis of sensuality
Today it is accepted to call cells (old) cells in which the cell cycle is stopped under the influence of various factors (stress or exhaustion of the division resource). As a result, these cells do not divide and are not updated.
At the Cell Senescence in Cancer and Aging conference held at the University of Cambridge, the definition of cell aging was given: “Cellular aging is called sustained proliferation arrest caused by various molecular triggers, including activation of oncogenes, as well as an excessive number of cell divisions. In addition, senescent cells are characterized by the secretion of a number of stromal regulators and regulators of inflammation (the so-called "associated with aging seroretny phenotype"), affecting the functioning of neighboring cells, including immunocompetent. A number of convincing evidence suggests that cellular aging is an effective mechanism for suppressing tumor growth. At the same time, cellular aging probably contributes to the aging of tissues and the whole body . ”
Due to the different causal mechanisms, there are three types of cellular aging.
The very first in the early 60s of the last century, cellular replicative aging was detected. In the already famous work, American gerontologists L. Hayflik and P. Moorhead, in experiments with cultured human fibroblasts, established that cells do not divide indefinitely and there is a limit to cell division (later called the Hayflick limit) [1]. After 10 years, Soviet biologist Alexei Olovnikov gave a logical explanation for this phenomenon by associating the limit of cell division with the gradual shortening of the end sections of DNA, telomeres. This is due to the fact that the enzyme telomerase, capable of increasing telomeres after shortening, is not active in most somatic cells. After telomeres are shortened to a critical level, a DNA damage response (DDR) response occurs, as a result, the cell cycle is stopped and the cell goes into the category of senescent. It is known that external factors that adversely affect health and longevity (obesity, lack of exercise, stress) also have a negative effect on telomere shortening [2]. Also, acceleration of telomere length reduction is observed in patients with neurodegenerative diseases, such as Alzheimer's disease [3].
It is believed that for most cells the Hayflick limit is about 50 divisions, after which the cell stops dividing. To distinguish the aging of the organism as a whole from cellular aging, Hayflik and Moorhead introduced a special term for cell aging, senescence (unlike aging of the organism, aging).
In addition to replicative aging, cell aging can also be caused by other factors that prematurely induce cell aging, regardless of telomere length. These factors constitute the second and third types of cellular aging.
Thus, the activation of oncogenes, such as RAS and RAF, causes cell aging, called oncogene-induced cell aging (oncogene-induced senescence, OIS). This form of cellular aging is associated with tumor suppression. Genomic comparative studies of cells with replicative and OIS aging show that, although there are some general changes in gene expression between these two species as compared to proliferating cells, there are also significant differences [4]. It is known that DNA damage associated with reactive oxygen species (ROS) play an important role in the mechanisms of OIS-aging. ERK kinase is also actively involved in the occurrence of OIS, stimulating the degradation of proteins necessary for the progression of the cell cycle. The role of the response to DNA damage (DDR) in this type of cellular aging is not done in Donets. Known that mutant oncogenes, such as H-Ras G12V, have the potential to activate molecular pathways of cellular aging associated with the p38 MAPK kinase and the transcription factor NF-kV, regardless of DNA damage. The oncogenic gene Ras can also contribute to an increase in p53 regulation through p19ARF and cell aging, regardless of DNA damage. [five]. Therefore, stimulation of cellular OIS aging is not excluded, even in the absence of DNA damage.
The third type of cellular aging, also independent of telomere length, is stress-induced premature cell aging (stress-induced premature senescence, SIPS). It arises in response to stress factors of different nature: ionizing and ultraviolet radiation, increasing the level of ROS, chemotherapeutic drugs. Unlike OIS-aging, the occurrence of SIPS is completely dependent on the response to DNA damage (DDR). Phenotypically, SIPS and replicative cell senescence are similar in many respects, but may vary at the level of protein expression. The role of SIPS in the general aging of the organism remains not fully clear - increased expression of antioxidants and suppression of ROS, the main factors responsible for the onset of SIPS, did not lead to an increase in the lifetime [6].
The molecular mechanisms of cell cycle arrest in senescent cells are being actively studied today. It is known that the degree of DNA damage affects the cell cycle in different ways. Thus, moderate DNA damage can induce a temporary stunted growth, extensive DNA damage causes programmed cell death, persistent DNA damage causes cell aging. Molecular determinants (major factors) that regulate the transition from a temporary stunted growth to irreversible cycle arrest are complex and not yet fully described. DNA damage is known to initially activate the p53-p21 pathway, which stops the cell cycle. Then, if the DNA damage is not repaired, the cell either goes into apoptosis, or becomes senescent. In the second case, the key role is played by the protein p16 INK4a,
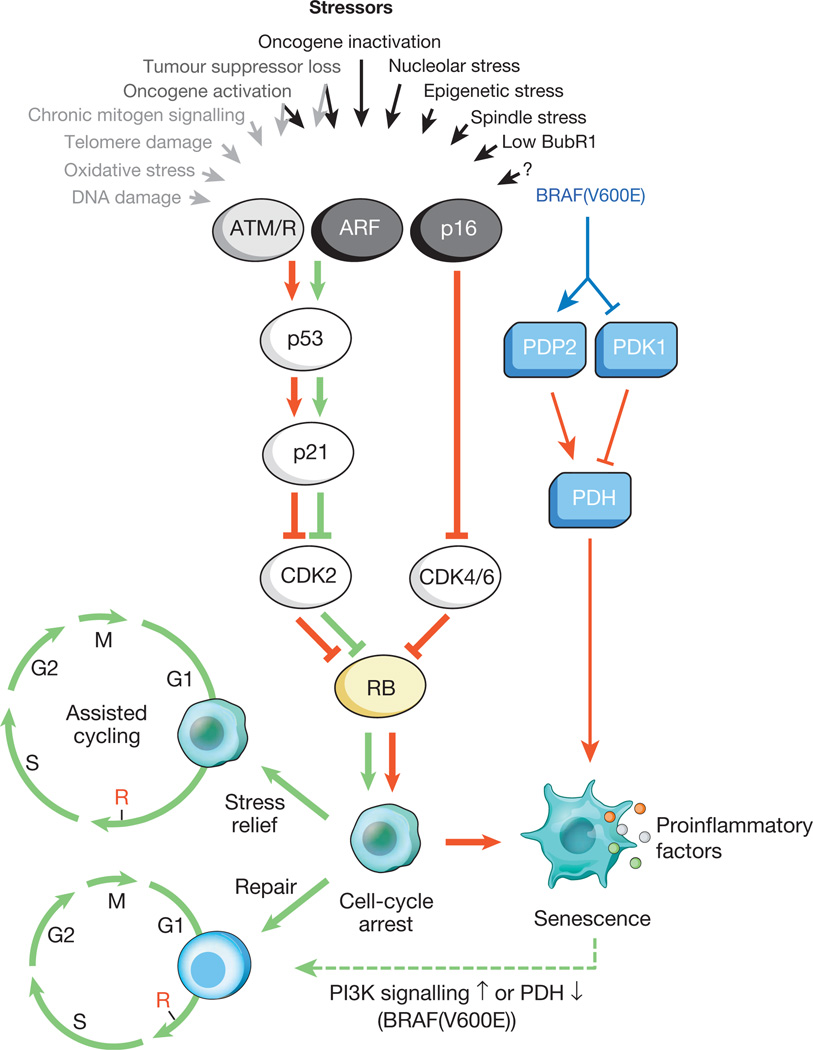
Fig.1. Incentives for cellular aging and major effector pathways
A variety of intracellular and external stresses can activate the program of cellular aging. These stressors capture various cellular signaling cascades, and eventually activate p53 and p16 INK4a. The types of stress that activate p53 signaling through DDR are indicated by gray text and arrows (ROS) cause a response to DNA damage (DDR), disrupting gene transcription and DNA replication, as well as reducing telomeres). Activated p53 induces p21, which causes a temporary cessation of the cell cycle by inhibiting cyclin E-Cdk2. p16 INK4a also inhibits progression of the cell cycle, but does so by targeting the complexes of cyclin D-Cdk4 and cyclin D-Cdk6. Both p21 and p16 INK4a act by preventing the inactivation of Rb, which leads to continued repression of the E2F target genes necessary for the onset of S-phase. Under severe stress (red arrows), temporarily blocked cells move to the arrest stage of the cell cycle. Cells subject to minor damage can be successfully repaired and resume a normal cycle. Thus, the path of p53-p21 can either antagonize or synergize its action with p16 INK4a in old age, depending on the type and level of stress. BRAF (V600E) is associated with aging through the metabolic effector pathway. BRAF (V600E) activates PDH, inducing PDP2 and inhibiting the expression of PDK1, promoting a shift from glycolysis to oxidative phosphorylation, which creates aging redox stress. Cells that are aging, induce inflammatory transcriptome regardless of inducing stress-inducing aging (colored dots represent various SASP factors). The red and green arrows, respectively, indicate an activity that promotes aging and the "prevention of aging." The dashed green arrow denotes the mechanism of the “change of aging”.
It is known that senescent cells actively influence their microenvironment (surrounding tissues), secreting a number of active molecules: pro-inflammatory cytokines, chemokines growth factors, proteases (about 40 different types of molecules). These substances were combined into a single group - the secretory phenotype associated with cellular aging (senescence associated secretory phenotype, SASP). It is known that SASP factors are actively involved in tissue remodeling in embryonic development. Moreover, under their influence, both the mother's organism and the embryo are rebuilt. It is assumed that the evolutionary nature of SASP is associated with a number of defense mechanisms: tumor suppression, recovery from injury, and tissue regeneration.
The simplified physiological effect of SASP can be described as follows. Secreted pro-inflammatory molecules form around the senescent cells to be removed a focus of inflammation. What attracts in this place the cells of the immune system for the elimination of aging cells. Matrix metalloproteases (MMP-1, MMP-10, MMP-3) and serine proteases that enter the SASP remodel the extracellular matrix in order to facilitate the penetration of immune cells into aging cells. And, finally, secreted growth factors stimulate the reproduction of neighboring cells to replace remote senescent.
This is described as the SASP mechanism should work normally, in a young and healthy body. But with age and with deviations, its effectiveness can significantly decrease, which causes the accumulation of senescent cells in the tissues and, as a result, to a prolonged secretion of pro-inflammatory factors of SASP. That is accompanied by the appearance of foci of chronic inflammation. In addition, it is known that prolonged SASP activity acts as an infection on normal cells. The active molecules secreted by senescent cells enter the extracellular space and, acting on the adjacent normal cells, initiate cell cycle arrest and proliferation arrest. Which greatly accelerates the development of cellular aging in the tissues.
In addition, prolonged secretion of SASP factors by aging cells is associated with the development of age-related pathologies. Thus, the increased secretion of matrix metalloproteases by senescent cells stimulates the development of coronary heart disease, osteoporosis and osteoarthritis. Senescent smooth muscle cells are involved in the development of atherosclerosis, through the secretion of a large number of pro-inflammatory cytokines. Prolonged secretion of proinflammatory factor TNF-α by senescent T-cells is involved in processes associated with bone tissue dysfunction. In addition, it is known that increased levels of pro-inflammatory IL-6 are associated with insulin resistance, diabetes, atherosclerosis, and liver disease. To refer to all these processes associated with systemic chronic inflammation and aging, in which the key role is played by the factors SASP, a special term was introduced - inflammaging. In addition, the dual role of SASP in carcinogenesis was described - its tumor suppressor and tumor stimulating activity [8].
In connection with the dual role of cell aging described above in young and old age, some scientists consider cell aging as an example of evolutionary antagonistic pleiotropy, which is still jokingly formulated with the phrase “use now - pay later”. According to this theory, the processes that were fixed in evolution to increase the survival rate of young organisms may have accumulating harmful effects in older individuals. Thus, the mechanisms associated with senescent cells at a young age are involved in pregnancy, recovery from injuries, and protection from tumors. At a more advanced age, the same mechanisms cause systemic inflammation, tissue degeneration, and the development of pathologies [9].
The authors of the SENS concept among the pool of senescent cells of the body highlight two types associated with adipose tissue: preadipocytes and visceral adipose tissue cells. And this is not by chance, since today it is known that visceral fat is metabolically active and releases a number of active molecules - adipokines. Which, in turn, are associated with the development of a number of age-related pathologies (insulin resistance, diabetes, cardiovascular diseases). Also, the authors of SENS focus on age-related deterioration of the immune system. In their opinion, this is due to the overload of the body by senescent cells and, as a response, to the overproduction of killer T cells that destroy senescent cells to the detriment of other types of immune cells. What makes an aging body vulnerable by various infections.
Solving the problem of accumulation of senescent cells, the authors of SENS see in two ways. The first is the development of drugs that are toxic to old cells, or causing apoptosis, but harmless to healthy, normal cells. And the second direction is the search for immune system stimulators to selectively search for and kill aging target cells. The most likely way to selectively attack these abnormal cells, according to the developers of SENS, would be to use the distinctive molecules that are found on their surfaces. Indeed, different cell types have differences in their surfaces. Therefore, the first step is the identification and orientation of cell surface markers that are specific to the senescent cells to be removed. This strategy is not abstract, but is already the basis of some cancer treatments,
Studies have shown that senescent cells actually accumulate with age in various tissues [11]. It was also described that the elimination of senescent cells, which accumulate in the model of rapidly aging mice, prevents the emergence of three main aging phenotypes (cataract, sarcopenia, loss of subcutaneous fat) [12]. In this regard, it is quite obvious that there is a need to identify reliable and effective biomarkers of cellular aging. Which are necessary first of all, for tracking of action of potential drugs-senolitikov.
The most commonly used biomarker of senescent cells is β-galactosidase (senescence-associated beta-galactosidase, SA-β-Gal) associated with cellular aging. The β-galactosidase enzyme is a lysosomal hydrolase that cleaves terminal beta-galactose from its compounds (lactose, keratin sulfates, sphingolipids, etc.). As early as 1995, it was described that the expression of SA-β-Gal increases significantly in senescent cells. An immunohistochemical method is used to determine its content in aging tissues. As an alternative method for determining SA-β-gal activity in cells, flow cytometry is used using 5-dodecanoyl-amino-fluorescein di-β-D-galactopyranoside as a substrate.
However, the use of SA-β-Gal as a biomarker of cellular aging has its limitations, since this enzyme can give false positives, increasing expression not only in aging cells, but also in “young” cells, which for various reasons have a restriction on proliferation. Therefore, today it is considered appropriate to use SA-β-Gal along with other markers of cellular aging.
In 2017, Israeli cytology developed a new, more efficient flow cytometry technology using the ImageStreamX cytometer. This method made it possible to detect SA-β-Gal in tissues with an efficiency of more than 80%. To improve the efficiency of the analysis, scientists along with SA-β-Gal have identified several more biomarkers of cellular aging - proteins HMGB1 and γH2AX. HMGB1 is a protein from the group of nuclear nonhistone proteins HMG, in senescent cells it leaves the nucleus and moves into the extracellular space. γH2AX, a phosphorylated form of histone H2AX, is a recognized marker of early DNA damage and cellular aging. In addition, the new method of Israeli scientists allowed to determine the aging cells by their increased size. According to researchers, their technology can be used to quickly determine the effectiveness of new pharmaceutical compounds that will be specifically designed to eliminate aging cells from tissues. [13].
Heterochromatin foci (SAHF) associated with cellular aging can be another biomarker of aging. SAHF are special heterochromatic structures that are formed in the nuclei of senescent cells. Their formation is associated with irreversible heterochromatinization associated with the inactivation of genes involved in this cell cycle (MCM3, PCNA, Cyclin A) located on this site. SAHF can be seen under a microscope after staining with a special DAPI dye. In addition, an increased expression of promyelocytic leukemia protein (PML) in senescent cells has been described, which can also be an additional marker of cellular aging [14].
American researchers have described the p16 INK4a protein from human peripheral blood T cells as a cell aging biomarker. p16 INK4a as already described above, takes the most active part in stopping the cell cycle of senescent cells. The expression of p16 INK4a increased in senescent cells and, as it turned out, was significantly associated with smoking and physical inactivity. In addition, expression of p16 INK4a was associated with plasma IL-6 concentration, a marker of age-related inflammation. According to scientists, the expression of p16 INK4a is an easily measured peripheral blood biomarker for determining cellular aging [15].
Appendix 1.
Cellular Aging Biomarkers.
1. Associated with cellular aging β-galactosidase (senescence-associated beta-galactosidase, SA-β-Gal).
2. HMGB1 protein (high-mobility group protein B1).
3. Phosphorylated histone γH2AX.
4. Associated with cellular aging heterochromatic foci (SAHF).
5. Promyelocytic leukemia protein (PML).
6. Protein p16 INK4a.
Author: Alexey Rzheshevsky.
At the Cell Senescence in Cancer and Aging conference held at the University of Cambridge, the definition of cell aging was given: “Cellular aging is called sustained proliferation arrest caused by various molecular triggers, including activation of oncogenes, as well as an excessive number of cell divisions. In addition, senescent cells are characterized by the secretion of a number of stromal regulators and regulators of inflammation (the so-called "associated with aging seroretny phenotype"), affecting the functioning of neighboring cells, including immunocompetent. A number of convincing evidence suggests that cellular aging is an effective mechanism for suppressing tumor growth. At the same time, cellular aging probably contributes to the aging of tissues and the whole body . ”
Due to the different causal mechanisms, there are three types of cellular aging.
The very first in the early 60s of the last century, cellular replicative aging was detected. In the already famous work, American gerontologists L. Hayflik and P. Moorhead, in experiments with cultured human fibroblasts, established that cells do not divide indefinitely and there is a limit to cell division (later called the Hayflick limit) [1]. After 10 years, Soviet biologist Alexei Olovnikov gave a logical explanation for this phenomenon by associating the limit of cell division with the gradual shortening of the end sections of DNA, telomeres. This is due to the fact that the enzyme telomerase, capable of increasing telomeres after shortening, is not active in most somatic cells. After telomeres are shortened to a critical level, a DNA damage response (DDR) response occurs, as a result, the cell cycle is stopped and the cell goes into the category of senescent. It is known that external factors that adversely affect health and longevity (obesity, lack of exercise, stress) also have a negative effect on telomere shortening [2]. Also, acceleration of telomere length reduction is observed in patients with neurodegenerative diseases, such as Alzheimer's disease [3].
It is believed that for most cells the Hayflick limit is about 50 divisions, after which the cell stops dividing. To distinguish the aging of the organism as a whole from cellular aging, Hayflik and Moorhead introduced a special term for cell aging, senescence (unlike aging of the organism, aging).
In addition to replicative aging, cell aging can also be caused by other factors that prematurely induce cell aging, regardless of telomere length. These factors constitute the second and third types of cellular aging.
Thus, the activation of oncogenes, such as RAS and RAF, causes cell aging, called oncogene-induced cell aging (oncogene-induced senescence, OIS). This form of cellular aging is associated with tumor suppression. Genomic comparative studies of cells with replicative and OIS aging show that, although there are some general changes in gene expression between these two species as compared to proliferating cells, there are also significant differences [4]. It is known that DNA damage associated with reactive oxygen species (ROS) play an important role in the mechanisms of OIS-aging. ERK kinase is also actively involved in the occurrence of OIS, stimulating the degradation of proteins necessary for the progression of the cell cycle. The role of the response to DNA damage (DDR) in this type of cellular aging is not done in Donets. Known that mutant oncogenes, such as H-Ras G12V, have the potential to activate molecular pathways of cellular aging associated with the p38 MAPK kinase and the transcription factor NF-kV, regardless of DNA damage. The oncogenic gene Ras can also contribute to an increase in p53 regulation through p19ARF and cell aging, regardless of DNA damage. [five]. Therefore, stimulation of cellular OIS aging is not excluded, even in the absence of DNA damage.
The third type of cellular aging, also independent of telomere length, is stress-induced premature cell aging (stress-induced premature senescence, SIPS). It arises in response to stress factors of different nature: ionizing and ultraviolet radiation, increasing the level of ROS, chemotherapeutic drugs. Unlike OIS-aging, the occurrence of SIPS is completely dependent on the response to DNA damage (DDR). Phenotypically, SIPS and replicative cell senescence are similar in many respects, but may vary at the level of protein expression. The role of SIPS in the general aging of the organism remains not fully clear - increased expression of antioxidants and suppression of ROS, the main factors responsible for the onset of SIPS, did not lead to an increase in the lifetime [6].
The molecular mechanisms of cell cycle arrest in senescent cells are being actively studied today. It is known that the degree of DNA damage affects the cell cycle in different ways. Thus, moderate DNA damage can induce a temporary stunted growth, extensive DNA damage causes programmed cell death, persistent DNA damage causes cell aging. Molecular determinants (major factors) that regulate the transition from a temporary stunted growth to irreversible cycle arrest are complex and not yet fully described. DNA damage is known to initially activate the p53-p21 pathway, which stops the cell cycle. Then, if the DNA damage is not repaired, the cell either goes into apoptosis, or becomes senescent. In the second case, the key role is played by the protein p16 INK4a,

Fig.1. Incentives for cellular aging and major effector pathways
A variety of intracellular and external stresses can activate the program of cellular aging. These stressors capture various cellular signaling cascades, and eventually activate p53 and p16 INK4a. The types of stress that activate p53 signaling through DDR are indicated by gray text and arrows (ROS) cause a response to DNA damage (DDR), disrupting gene transcription and DNA replication, as well as reducing telomeres). Activated p53 induces p21, which causes a temporary cessation of the cell cycle by inhibiting cyclin E-Cdk2. p16 INK4a also inhibits progression of the cell cycle, but does so by targeting the complexes of cyclin D-Cdk4 and cyclin D-Cdk6. Both p21 and p16 INK4a act by preventing the inactivation of Rb, which leads to continued repression of the E2F target genes necessary for the onset of S-phase. Under severe stress (red arrows), temporarily blocked cells move to the arrest stage of the cell cycle. Cells subject to minor damage can be successfully repaired and resume a normal cycle. Thus, the path of p53-p21 can either antagonize or synergize its action with p16 INK4a in old age, depending on the type and level of stress. BRAF (V600E) is associated with aging through the metabolic effector pathway. BRAF (V600E) activates PDH, inducing PDP2 and inhibiting the expression of PDK1, promoting a shift from glycolysis to oxidative phosphorylation, which creates aging redox stress. Cells that are aging, induce inflammatory transcriptome regardless of inducing stress-inducing aging (colored dots represent various SASP factors). The red and green arrows, respectively, indicate an activity that promotes aging and the "prevention of aging." The dashed green arrow denotes the mechanism of the “change of aging”.
It is known that senescent cells actively influence their microenvironment (surrounding tissues), secreting a number of active molecules: pro-inflammatory cytokines, chemokines growth factors, proteases (about 40 different types of molecules). These substances were combined into a single group - the secretory phenotype associated with cellular aging (senescence associated secretory phenotype, SASP). It is known that SASP factors are actively involved in tissue remodeling in embryonic development. Moreover, under their influence, both the mother's organism and the embryo are rebuilt. It is assumed that the evolutionary nature of SASP is associated with a number of defense mechanisms: tumor suppression, recovery from injury, and tissue regeneration.
The simplified physiological effect of SASP can be described as follows. Secreted pro-inflammatory molecules form around the senescent cells to be removed a focus of inflammation. What attracts in this place the cells of the immune system for the elimination of aging cells. Matrix metalloproteases (MMP-1, MMP-10, MMP-3) and serine proteases that enter the SASP remodel the extracellular matrix in order to facilitate the penetration of immune cells into aging cells. And, finally, secreted growth factors stimulate the reproduction of neighboring cells to replace remote senescent.
This is described as the SASP mechanism should work normally, in a young and healthy body. But with age and with deviations, its effectiveness can significantly decrease, which causes the accumulation of senescent cells in the tissues and, as a result, to a prolonged secretion of pro-inflammatory factors of SASP. That is accompanied by the appearance of foci of chronic inflammation. In addition, it is known that prolonged SASP activity acts as an infection on normal cells. The active molecules secreted by senescent cells enter the extracellular space and, acting on the adjacent normal cells, initiate cell cycle arrest and proliferation arrest. Which greatly accelerates the development of cellular aging in the tissues.
In addition, prolonged secretion of SASP factors by aging cells is associated with the development of age-related pathologies. Thus, the increased secretion of matrix metalloproteases by senescent cells stimulates the development of coronary heart disease, osteoporosis and osteoarthritis. Senescent smooth muscle cells are involved in the development of atherosclerosis, through the secretion of a large number of pro-inflammatory cytokines. Prolonged secretion of proinflammatory factor TNF-α by senescent T-cells is involved in processes associated with bone tissue dysfunction. In addition, it is known that increased levels of pro-inflammatory IL-6 are associated with insulin resistance, diabetes, atherosclerosis, and liver disease. To refer to all these processes associated with systemic chronic inflammation and aging, in which the key role is played by the factors SASP, a special term was introduced - inflammaging. In addition, the dual role of SASP in carcinogenesis was described - its tumor suppressor and tumor stimulating activity [8].
In connection with the dual role of cell aging described above in young and old age, some scientists consider cell aging as an example of evolutionary antagonistic pleiotropy, which is still jokingly formulated with the phrase “use now - pay later”. According to this theory, the processes that were fixed in evolution to increase the survival rate of young organisms may have accumulating harmful effects in older individuals. Thus, the mechanisms associated with senescent cells at a young age are involved in pregnancy, recovery from injuries, and protection from tumors. At a more advanced age, the same mechanisms cause systemic inflammation, tissue degeneration, and the development of pathologies [9].
The authors of the SENS concept among the pool of senescent cells of the body highlight two types associated with adipose tissue: preadipocytes and visceral adipose tissue cells. And this is not by chance, since today it is known that visceral fat is metabolically active and releases a number of active molecules - adipokines. Which, in turn, are associated with the development of a number of age-related pathologies (insulin resistance, diabetes, cardiovascular diseases). Also, the authors of SENS focus on age-related deterioration of the immune system. In their opinion, this is due to the overload of the body by senescent cells and, as a response, to the overproduction of killer T cells that destroy senescent cells to the detriment of other types of immune cells. What makes an aging body vulnerable by various infections.
Solving the problem of accumulation of senescent cells, the authors of SENS see in two ways. The first is the development of drugs that are toxic to old cells, or causing apoptosis, but harmless to healthy, normal cells. And the second direction is the search for immune system stimulators to selectively search for and kill aging target cells. The most likely way to selectively attack these abnormal cells, according to the developers of SENS, would be to use the distinctive molecules that are found on their surfaces. Indeed, different cell types have differences in their surfaces. Therefore, the first step is the identification and orientation of cell surface markers that are specific to the senescent cells to be removed. This strategy is not abstract, but is already the basis of some cancer treatments,
Studies have shown that senescent cells actually accumulate with age in various tissues [11]. It was also described that the elimination of senescent cells, which accumulate in the model of rapidly aging mice, prevents the emergence of three main aging phenotypes (cataract, sarcopenia, loss of subcutaneous fat) [12]. In this regard, it is quite obvious that there is a need to identify reliable and effective biomarkers of cellular aging. Which are necessary first of all, for tracking of action of potential drugs-senolitikov.
The most commonly used biomarker of senescent cells is β-galactosidase (senescence-associated beta-galactosidase, SA-β-Gal) associated with cellular aging. The β-galactosidase enzyme is a lysosomal hydrolase that cleaves terminal beta-galactose from its compounds (lactose, keratin sulfates, sphingolipids, etc.). As early as 1995, it was described that the expression of SA-β-Gal increases significantly in senescent cells. An immunohistochemical method is used to determine its content in aging tissues. As an alternative method for determining SA-β-gal activity in cells, flow cytometry is used using 5-dodecanoyl-amino-fluorescein di-β-D-galactopyranoside as a substrate.
However, the use of SA-β-Gal as a biomarker of cellular aging has its limitations, since this enzyme can give false positives, increasing expression not only in aging cells, but also in “young” cells, which for various reasons have a restriction on proliferation. Therefore, today it is considered appropriate to use SA-β-Gal along with other markers of cellular aging.
In 2017, Israeli cytology developed a new, more efficient flow cytometry technology using the ImageStreamX cytometer. This method made it possible to detect SA-β-Gal in tissues with an efficiency of more than 80%. To improve the efficiency of the analysis, scientists along with SA-β-Gal have identified several more biomarkers of cellular aging - proteins HMGB1 and γH2AX. HMGB1 is a protein from the group of nuclear nonhistone proteins HMG, in senescent cells it leaves the nucleus and moves into the extracellular space. γH2AX, a phosphorylated form of histone H2AX, is a recognized marker of early DNA damage and cellular aging. In addition, the new method of Israeli scientists allowed to determine the aging cells by their increased size. According to researchers, their technology can be used to quickly determine the effectiveness of new pharmaceutical compounds that will be specifically designed to eliminate aging cells from tissues. [13].
Heterochromatin foci (SAHF) associated with cellular aging can be another biomarker of aging. SAHF are special heterochromatic structures that are formed in the nuclei of senescent cells. Their formation is associated with irreversible heterochromatinization associated with the inactivation of genes involved in this cell cycle (MCM3, PCNA, Cyclin A) located on this site. SAHF can be seen under a microscope after staining with a special DAPI dye. In addition, an increased expression of promyelocytic leukemia protein (PML) in senescent cells has been described, which can also be an additional marker of cellular aging [14].
American researchers have described the p16 INK4a protein from human peripheral blood T cells as a cell aging biomarker. p16 INK4a as already described above, takes the most active part in stopping the cell cycle of senescent cells. The expression of p16 INK4a increased in senescent cells and, as it turned out, was significantly associated with smoking and physical inactivity. In addition, expression of p16 INK4a was associated with plasma IL-6 concentration, a marker of age-related inflammation. According to scientists, the expression of p16 INK4a is an easily measured peripheral blood biomarker for determining cellular aging [15].
Appendix 1.
Cellular Aging Biomarkers.
1. Associated with cellular aging β-galactosidase (senescence-associated beta-galactosidase, SA-β-Gal).
2. HMGB1 protein (high-mobility group protein B1).
3. Phosphorylated histone γH2AX.
4. Associated with cellular aging heterochromatic foci (SAHF).
5. Promyelocytic leukemia protein (PML).
6. Protein p16 INK4a.
Author: Alexey Rzheshevsky.
Bibliography
1. L.HayflickP.S.Moorhead. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961 Dec;25:585-621.
2. Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004 Dec 7;101(49):17312-5.
3. Panossian LA, Porter VR, Valenzuela HF, Zhu X, Reback E, Masterman D, Cummings JL, Effros RB. Telomere shortening in T cells correlates with Alzheimer's disease status. Neurobiol Aging. 2003 Jan-Feb;24(1):77-84.
4. Nelson DM1, McBryan T, Jeyapalan JC, Sedivy JM, Adams PD. A comparison of oncogene-induced senescence and replicative senescence: implications for tumor suppression and aging. Age (Dordr). 2014 Jun;36(3):9637.
5. Palmero I, Pantoja C, Serrano M. p19ARF links the tumour suppressor p53 to Ras. Nature. 1998 Sep 10; 395(6698):125-6.
6. Pérez VI1, Van Remmen H, Bokov A, Epstein CJ, Vijg J, Richardson A. The overexpression of major antioxidant enzymes does not extend the lifespan of mice. Aging Cell. 2009 Feb;8(1):73-5.
7. Narita M, Nũnez S, Heard E, Narita M, Lin AW, Hearn SA, Spector DL, Hannon GJ, Lowe SW. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 2003 Jun 13; 113(6):703-16.
8. Бородкина А.В., Дерябин П.И., Грюкова А.А., Никольский Н.Н. «Социальная жизнь» стареющих клеток: что такое SASP и зачем его изучать? Acta Naturae, 2018, 10(1). С.4-15.
9. Shankar J. Chinta, Georgia Woods, Anand Rane, Marco Demaria, Judith Campisi, and Julie K Andersen. Cellular senescence and the aging brain. Exp Gerontol. 2015 Aug; 68: 3–7.
10. ApoptoSENS: Removing dysfunctional cells.
11. Jeyapalan JC, Ferreira M, Sedivy JM, Herbig U. Accumulation of senescent cells in mitotic tissue of aging primates. Mech Ageing Dev. 2007 Jan;128(1):36-44.
12. Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011 Nov 2;479(7372):232-6.
13. Anat Biran, Lior Zada, Paula Abou Karam, Ezra Vadai, Lior Roitman, Yossi Ovadya, Ziv Porat, and Valery Krizhanovsky. Quantitative identification of senescent cells in aging and disease. Aging Cell. 2017 Aug; 16(4): 661–671.
14. Bruno Bernardes de Jesus and Maria A. Blasco. Assessing Cell and Organ Senescence Biomarkers. Circ Res. 2012 Jun 22; 111(1): 97–109.
15. Yan Liu, Hanna K. Sanoff, Hyunsoon Cho, Christin E. Burd, Chad Torrice, Joseph G Ibrahim, Nancy E. Thomas, and Norman E. Sharpless. Expression of p16INK4a in peripheral blood T-cells is a biomarker of human aging. Aging Cell. 2009 Aug; 8(4): 439–448.
2. Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004 Dec 7;101(49):17312-5.
3. Panossian LA, Porter VR, Valenzuela HF, Zhu X, Reback E, Masterman D, Cummings JL, Effros RB. Telomere shortening in T cells correlates with Alzheimer's disease status. Neurobiol Aging. 2003 Jan-Feb;24(1):77-84.
4. Nelson DM1, McBryan T, Jeyapalan JC, Sedivy JM, Adams PD. A comparison of oncogene-induced senescence and replicative senescence: implications for tumor suppression and aging. Age (Dordr). 2014 Jun;36(3):9637.
5. Palmero I, Pantoja C, Serrano M. p19ARF links the tumour suppressor p53 to Ras. Nature. 1998 Sep 10; 395(6698):125-6.
6. Pérez VI1, Van Remmen H, Bokov A, Epstein CJ, Vijg J, Richardson A. The overexpression of major antioxidant enzymes does not extend the lifespan of mice. Aging Cell. 2009 Feb;8(1):73-5.
7. Narita M, Nũnez S, Heard E, Narita M, Lin AW, Hearn SA, Spector DL, Hannon GJ, Lowe SW. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 2003 Jun 13; 113(6):703-16.
8. Бородкина А.В., Дерябин П.И., Грюкова А.А., Никольский Н.Н. «Социальная жизнь» стареющих клеток: что такое SASP и зачем его изучать? Acta Naturae, 2018, 10(1). С.4-15.
9. Shankar J. Chinta, Georgia Woods, Anand Rane, Marco Demaria, Judith Campisi, and Julie K Andersen. Cellular senescence and the aging brain. Exp Gerontol. 2015 Aug; 68: 3–7.
10. ApoptoSENS: Removing dysfunctional cells.
11. Jeyapalan JC, Ferreira M, Sedivy JM, Herbig U. Accumulation of senescent cells in mitotic tissue of aging primates. Mech Ageing Dev. 2007 Jan;128(1):36-44.
12. Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011 Nov 2;479(7372):232-6.
13. Anat Biran, Lior Zada, Paula Abou Karam, Ezra Vadai, Lior Roitman, Yossi Ovadya, Ziv Porat, and Valery Krizhanovsky. Quantitative identification of senescent cells in aging and disease. Aging Cell. 2017 Aug; 16(4): 661–671.
14. Bruno Bernardes de Jesus and Maria A. Blasco. Assessing Cell and Organ Senescence Biomarkers. Circ Res. 2012 Jun 22; 111(1): 97–109.
15. Yan Liu, Hanna K. Sanoff, Hyunsoon Cho, Christin E. Burd, Chad Torrice, Joseph G Ibrahim, Nancy E. Thomas, and Norman E. Sharpless. Expression of p16INK4a in peripheral blood T-cells is a biomarker of human aging. Aging Cell. 2009 Aug; 8(4): 439–448.
