Who said that Flabber is not real: creating a homogeneous thermosetting polymer gel

What things do you associate the modern world in terms of technology? Computers, robots, spacecraft, artificial organs, cloning, etc. Discoveries and researches in these and other areas are like bright flashes of fireworks - we see them, hear and wonder together, saying something like "wow" or "wow, how cool." And yes, when you read that in 5 years they plan to transplant artificial lungs to a person, you realize that we live in a wondrous world where everything previously impossible becomes real. However, today we will talk about technology, whose contribution to our life has become enormous, although now we take it for granted. It will be about artificial polymers. More precisely to say about the spontaneous synthesis of a homogeneous thermosetting polymer network. It will seem to someone that this is another difficult perceived scientific foul language, However, it’s not all that complicated and much more interesting. Let's see how scientists managed to create a new kind of polymers and what is special about them. Go.
Background
But for a start, a little excursion into history, or into terminology.
Polymers are not man-made substances, that is, they were not created by man in their original form. Polymers surrounded us long before the golden age of science or the industrial revolution. Proteins, polysaccharides, nucleic acids, etc. - all these are polymers, more precisely biopolymers. This is a class of polymers that are present in all living organisms, plants or animals. We ourselves are made up of biopolymers.
A striking example of biopolymers is the double helix of DNA.
A polymer is a substance that consists of units of monomers linked together in long macromolecules.If we talk about the relationship of man and polymer as an object of study, the story takes its origin quite a long time. Back in the middle of the XIX century, scientist Alexander Butlerov was the first to conduct an experiment with polymerization, thereby proving by experience that the structure of a molecule can be changed, while its composition and weight will remain the same. This work was a confirmation of his statements, which later grew into a “theory of chemical structure”.
Fundamentals of the theory of Butlerov:
- Based on their valency, the atoms in the molecules are connected to each other in a specific sequence. This sequence is referred to as chemical structure.
- The properties of a substance depend not only on which atoms form it, but also on the sequence of the combination of atoms in a molecule.
- Knowing the properties of a substance, one can determine its molecular structure. And vice versa.
- Atoms and their groups affect each other inside the molecule.

Alexander Mikhailovich Butlerov
Such a discovery was really a radically new look at the structure of substances and formed the foundation for the modern theory of chemical structure.
Later, in 1906, the American chemist Leo Bakeland received Bakelite resin, a condensation product of phenol and formaldehyde. This was the first appearance of a synthetic organic polymer.
Thus began the long and successful journey of synthetic polymers. In the course of time, more and more new types of them appeared, possessing more and more outstanding properties. Now, in order to demonstrate the prevalence of polymers, it is only necessary to look around. And it does not matter whether you are at home or on the street, artificial polymers are practically everywhere: stationery pens, computer cases, parts in cars, grocery bags, diapers and much more.
This discovery, even though we now take it for granted, once made the most real revolution in chemistry, which led to a revolution in a variety of technologies and in our lives in general.
Basics of research
This study focuses on a specific category of synthetic polymers - thermosetting. In contrast, there are thermoplastic polymers.
The molecules of thermosetting polymers have a linear structure, as do the molecules of thermoplastic polymers. However, there is an important difference: the molecules of the first are able to join into groups. At a certain effect, as a rule, heating, a continuous (homogeneous) spatial network is formed, thus the molecular structure of the thermosetting polymer becomes nonlinear.
By the way, bakelite resin, a short one we mentioned earlier, refers specifically to thermosetting polymers.
An important difference between thermoplastic and thermosetting polymers is also the fact that during heating the first softens and melts, and when cooled, hardens. But the thermosetting polymer undergoes irreversible chemical destruction when heated, but does not melt.
Thermosetting polymers have a number of advantages (low price, resistance to external factors, etc.) and, of course, disadvantages (toxicity, fragility, long process of formation, etc.).
It is about the creation of a homogeneous thermosetting polymer and will be discussed in today's study. Scientists decided to improve this process by simplifying it. The method of creating a network structure of monodisperse polymers *really simple - mixing the necessary compounds. In other words, chemical vinaigrette, the ingredients of which you experimentally select to achieve the perfect "taste" at the exit.
Monodisperse polymers * - polymers consisting of identical macromolecules.To achieve this goal, scientists have combined polymerization * with a high reaction rate and crosslinking * with a low (compared to polymerization) reaction rate. The important point is that both reactions have a common catalyst.
Polymerization * is the process of creating a high molecular weight compound when monomer molecules are successively attached to the active center at the end of the growing chain, which forms the polymer molecule.
Stitching * - the reaction of the formation of transverse chemical bonds of macromolecules with the formation of a spatial network.Scientists decided to use the workings of their colleagues and predecessors in their work. In particular, radical polymerization in the mode of living chains, when it is possible in a short time to get a variety of monodisperse polymers required for this study.
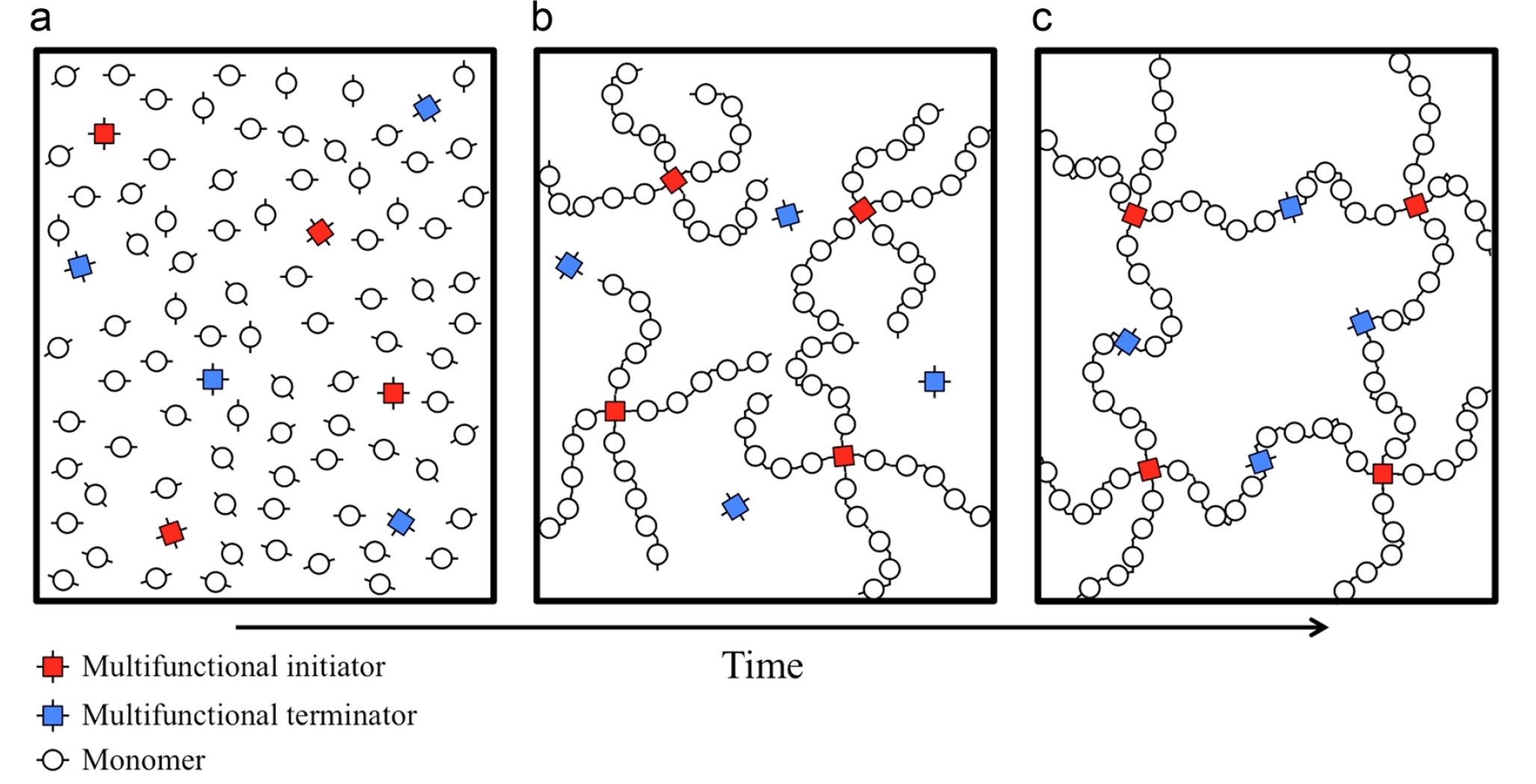
Polymer creation scheme
In the course of the experiments, a polymer mesh sample was obtained, consisting of polymers with a small MMD * , using the methods of a multifunctional initiator * and a multifunctional terminator * .
MMP * - the molecular weight distribution is the ratio of macromolecules of different molecular weights in a polymer.
Initiator * - a substance easily decomposing into free radicals. It is used to initiate radical polymerization by adding this substance (no more than 1% by weight of the monomer).
Terminator * - a substance that is the basis of the termination reaction, when the formation of reactive intermediates stops at the stage of chain creation during the polymerization process.In this experiment, for termination, polymers with a small MWD were prepared in advance by radical polymerization in the mode of living chains. Further, these polymers were combined with several polymer chains to form a star-shaped polymer (scheme above, image c ). After mixing the necessary "ingredients", two reactions with different speeds (mentioned earlier) occur sequentially, which led to the formation of a polymer gel with a relatively homogeneous network. structure.
The results of the experiments
DMF / H 2 O: evaluation of the optimal ratio of components
During the polymerization of vinyl monomers using copper (as a catalyst) and a halogenated organic compound (as an initiator) in a solution of dimethylformamide *(DMF) and water there is a disproportionation reaction * of copper, which leads to a radical polymerization in the mode of living chains, initiated by electron transfer.
Dimethylformamide * - (CH 3 ) 2 NC (O) H is an organic substance, which in this case is used as a solvent in the creation of the polymer.
Disproportionation * is a chemical reaction when one element acts as an oxidizing agent and a reducing agent at the same time.This technique allows you to get enough of the desired polymer in a short time.
The use of radical polymerization in the mode of living chains initiated by electron transfer on a multifunctional initiator (tetra pentaerythritol 2-chloropropionate - C 37 H 68 O 8 ) allowed synthesizing a star-shaped polymer from four monodisperse polymers.
In order to test how the composition of the solution affects the polymerization process, C 37 H 68 O 8 was used as an initiator in a solution of dimethylformamide (DMF) and water (H 2 O) when the proportion of DMF was 25, 50 or 75% by volume. In addition, N-isopropylacrylamide was used as the vinyl monomer ((C6 H 11 NO) n ) to form a thermosetting polymer. The polymerization temperature was 4 ° C.
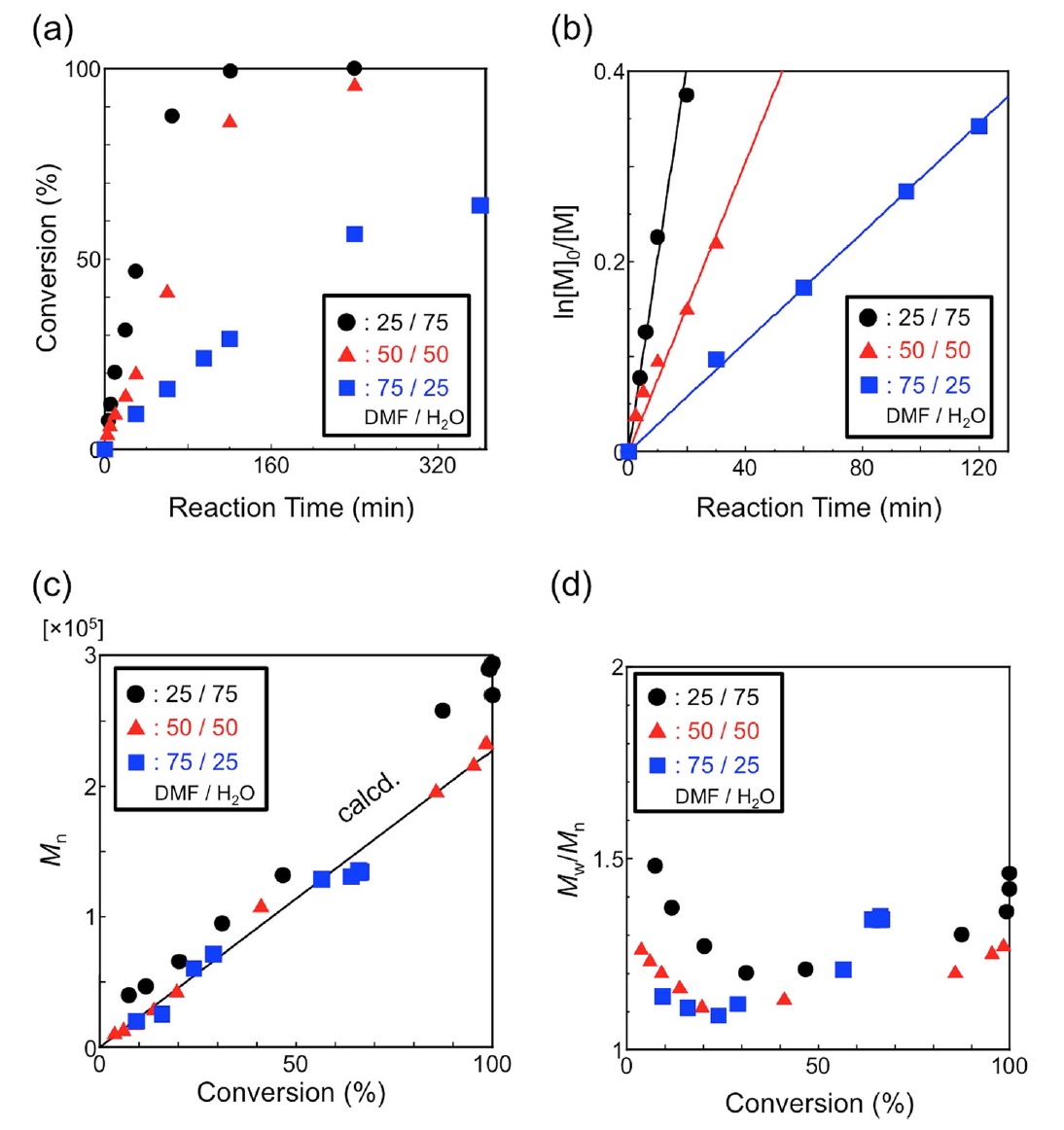
Image No. 1:
a) - diagram of the process of transformation of the monomer into the polymer, depending on the reaction time;
b) - consideration of polymerization as a primary reaction, based on diagram a;
c) is the ratio of the average molecular weight of the polymer obtained and the conversion (conversion of monomer to polymer);
d) - the relationship between molecular weight distribution (MMP) and conversion.
The graphs above, together, demonstrate the polymerization process, where C 37 H 68 O 8 , (C 6H 11 NO) n and CuCl (copper chloride) act as a catalyst, and C 12 H 30 N 4 as their ligand * . Both the catalyst and the ligand were dissolved in a solvent system with a different DMF / H 2 O composition in argon.
A ligand * is a molecule, atom or ion associated with a certain center.After mixing all the components, the polymerization process of N-isopropylacrylamide continued. When the proportion of DMF reached 25%, almost all of the N-isopropylacrylamide was used after 2 hours.
With a DMF concentration of 50%, the process slowed slightly, but about 95% of N-isopropylacrylamide was used after 4 hours. A significant drop in this indicator (67% in 24 hours) was observed already with a DMF share of 75%.
Analysis of the data showed that in order to achieve the best polymerization result, the proportion of DMF should be from 25 to 50% by volume. For already at 60% by volume, the MMP increases in the second half of the process, and at 75% by volume, the whole process becomes much more complicated and slows down.
The influence of the composition of the solvent and temperature on the process of swelling of the polymer gel
Polymers have the property to swell, that is, to increase their volume due to the absorption of a liquid, while maintaining its property of non-flowability.
In this case, it is extremely important that the solvent was of adequate quality, so to speak. Since during the synthesis of the gel in such solvents, the polymer aggregates and precipitates, and this does not allow the formation of a polymer gel with a homogeneous network structure. That is, the result of this action will be completely opposite to the expected.
For the experiments, a cylindrical gel-polymer, made by means of classical radical polymerization with methylene bisacrylamide (C7H10N2O2) as a "crosslinking" agent, was used.
This sample gel was made in water at 4 ° C. This temperature is not accidental. At 4 ° C, water becomes an excellent solvent for the polymer being tested. Consequently, in addition to the ratio of solvent components, temperature also plays an important role.
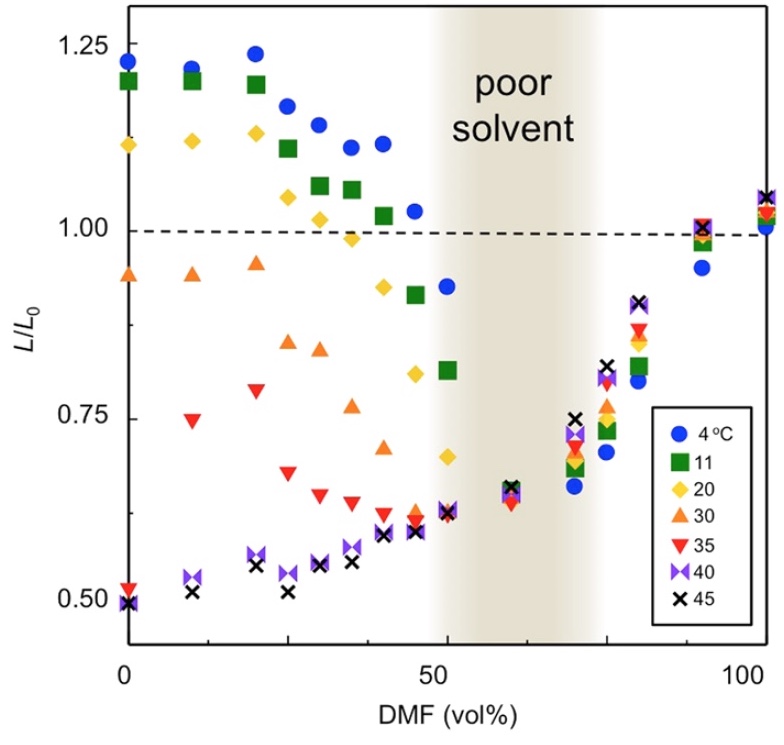
Image №2
The graph above shows the degree of swelling of the polymer gel at different temperatures and the solvent DMF / H 2 O with a different composition. The degree of swelling ( L / L0 ) is the ratio of the length of the polymer gel at the time of preparation ( L0 ) and in certain conditions ( L ), given experimentally.
At temperatures ≤ 35 ° C and ≤ 50 vol.% DMF, if the volume of water increases, the gel begins to actively swell, but as the temperature rises to 40 ° C, it shrinks.
If the vol.% DMF is equal to or greater than 50, the effect of temperature becomes less noticeable, but swelling does occur if the volume of DMF is very large.
When the swelling index L / L0 ≤ 1, it becomes impossible to obtain the desired polymer gel, that is, this indicator should be equal to or greater than 1. And this corresponds to such conditions: the temperature is not higher than 20 ° C, and the volume percentage of DMF in the solvent is not higher than 50% .
Creating a homogeneous polymer gel
In addition to the above-described compounds, tetraallylglycol aurol (TA-G), a component from the allyl group of radicals, was used in the preparation of the future polymer gel.
During the test, the viscosity increased gradually, but the test mixture remained in solution until the mark at 5 o'clock after the start of the test. At this point, C 37 H 68 O 8 activates the polymerization. However, with a DMF volume fraction of 25 vol.%, The sample freezes only after 6 hours.
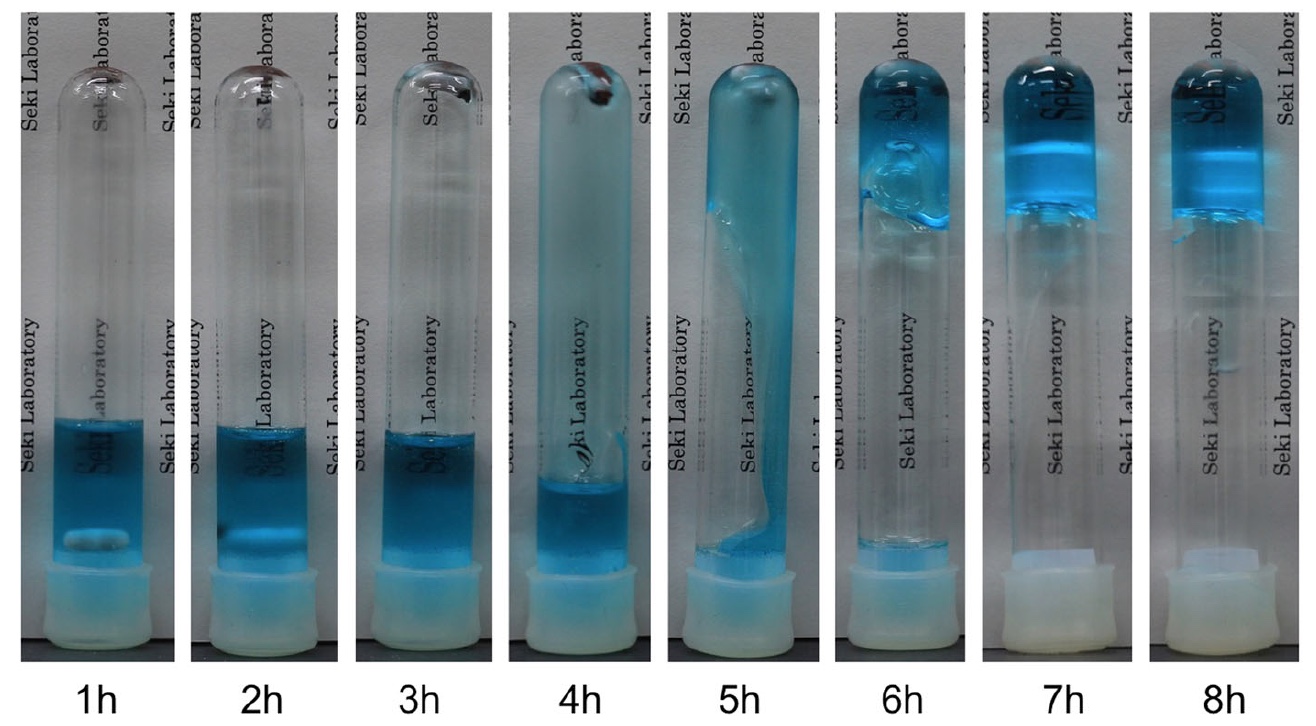
Image No. 3: the process of freezing the gel at 25 vol.% DMF and a temperature of 4 ° C.
In this case, TA-G acts as a terminator, completing the polymerization process, thereby forming a polymer gel. To achieve this result, 5 equivalents of TA-G in C 37 H 68 O 8 were added .
If only 1 equivalent of TA-G was used, the viscosity increased, but freezing was never observed. At 2 TA-G - partial viscosity was observed, at 3 or more - full, and at 5 - a transparent and elastic polymer gel was obtained.
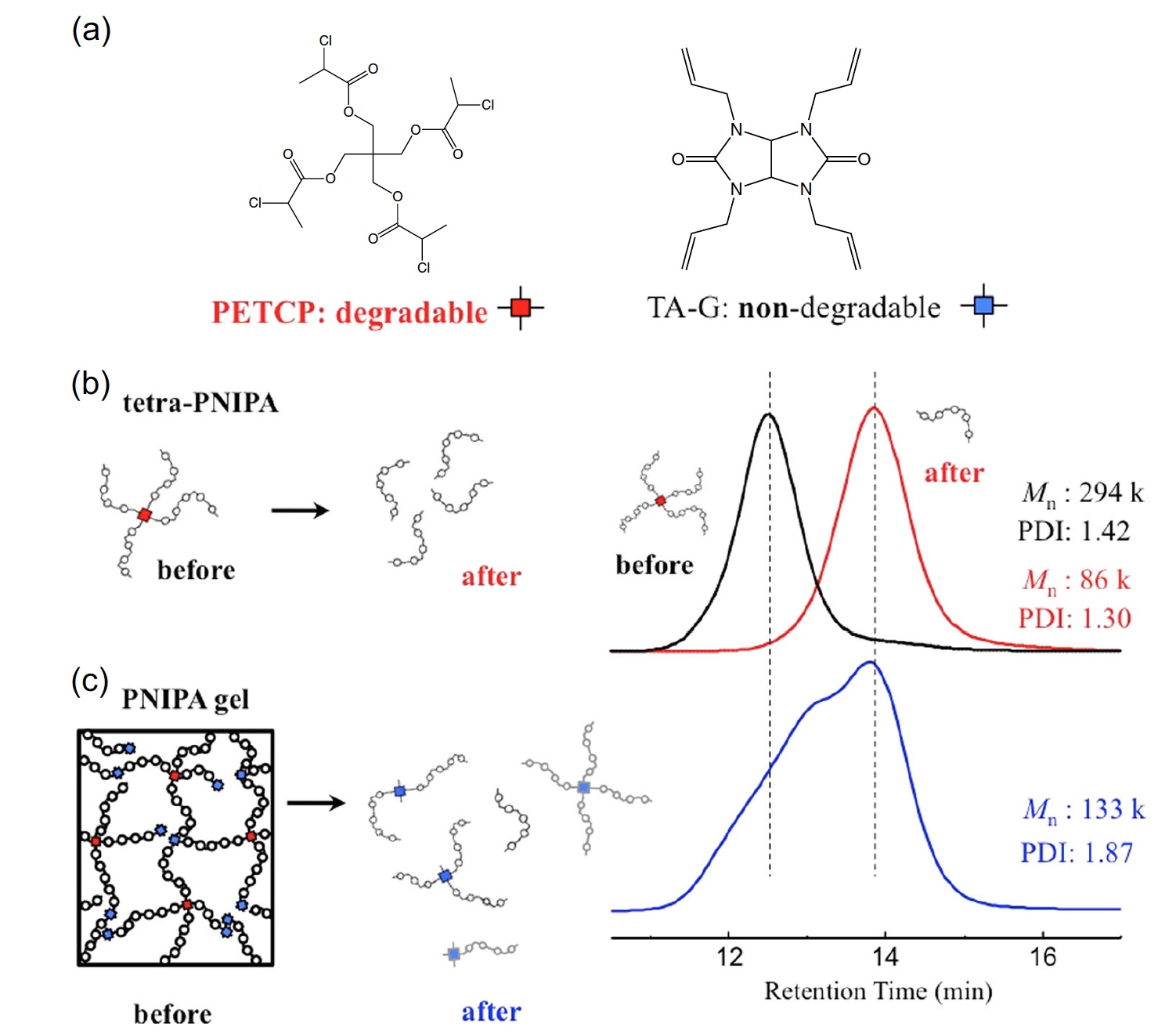
Image No. 4
Image 4a shows the chemical structures of C 37 H 68 O 8 and TA-G. 4b is the exclusion chromatography of a polymer obtained by hydrolysis of a star polymer. 4c shows the results of size exclusion chromatography of a polymer obtained by hydrolysis of a polymer network.
The video above shows 2 samples of polymer gel: experimental (blue) and obtained by conventional radical polymerization (white). As we can see, the difference is quite obvious. ( Download link (automatic) video ) The
report of scientists on this study is available here .
And additional materials to him - here .
Epilogue
Scientists have managed to create a new type of homogeneous thermosetting polymer gel with outstanding properties. In addition, the manufacturing method was also improved by them so that a large amount of gel could be created in a short time.
This material is definitely its application in a variety of technologies, from medicine to space exploration. There is no doubt about this, but the researchers are not going to rush, because this discovery needs some work, despite the surprising results.
Given this research, as well as work in the field of artificial intelligence, the use of bacteria in various technologies, etc., you begin to think that Flabber (1997 comedy) is not such a science fiction.
Thank you for staying with us. Do you like our articles? Want to see more interesting materials? Support us by placing an order or recommending to your friends, a 30% discount for Habr's users for a unique analogue of the entry-level servers that we invented for you:The whole truth about VPS (KVM) E5-2650 v4 (6 Cores) 10GB DDR4 240GB SSD 1Gbps from $ 20 or how to share the server? (Options are available with RAID1 and RAID10, up to 24 cores and up to 40GB DDR4).
VPS (KVM) E5-2650 v4 (6 Cores) 10GB DDR4 240GB SSD 1Gbps until December for free if you pay for a period of six months, you can order here .
Dell R730xd 2 times cheaper? Only we have 2 x Intel Dodeca-Core Xeon E5-2650v4 128GB DDR4 6x480GB SSD 1Gbps 100 TV from $ 249 in the Netherlands and the USA! Read about How to build an infrastructure building. class c using servers Dell R730xd E5-2650 v4 worth 9000 euros for a penny?
