New way to create nanotubes: now in color
Carbon nanotubes have become an integral part of modern technology. Their mechanical and electronic properties, as well as nanometer dimensions served this. This material is used in many areas: from batteries to displays. The quality of nanotubes, to a greater extent, depends on the chirality index (when there is no symmetry between the right and left sides). The smaller this indicator, the better the nanotube will be. There are several options for creating nanotubes, and they all work. But this does not mean that some enthusiasts will not try to invent their own new way that will be better than their predecessors. This is what the research will be about, in which we will deal with you. Go.
Prehistory
For a start, in brief, remember that there is a carbon nanotube. This can be simply understood by the name of this material. First, it is a cylindrical structure (tube) of graphite planes, the dimensions of which can be of the order of several nanometers. There are two main types of nanotubes: single-walled and multi-walled (image below).

In today's study we will focus on single. In order to have a new method for creating nanotubes with which to compare, the researchers cite as an example several already existing methods that allow achieving a low index of chirality distribution, which is extremely important for nanotubes. The first method - post-synthetic processing - is most often based on such techniques:
- ion exchange chromatography * (IEX) of single-wall nanotubes twisted like DNA;
- centrifugation in a density gradient * (DGU);
- size exclusion chromatography * ;
- two-phase water separation * .
Ion exchange chromatography * is a method of separating ions and polar molecules based on the charges of the separated molecules.All of the above techniques are somehow related to the dissolution of something in something. The researchers believe that this is a big problem, because in the process of dissolving the sample may be contaminated. And this will negatively affect the quality of the nanotube, and as a result, on its properties.
Centrifugation in a density gradient * - separation of macromolecules on the basis of their distribution in different parts of the gradient density.
Exclusive chromatography * - separation of molecules by size due to their distinct ability to penetrate the pores of the solid phase (or liquid) bound on an inert carrier.
Two-phase water separation * - the distribution of particles between the phases of a two-phase aqueous system.
The second method is the direct cultivation of single-walled nanotubes. Which, according to scientists, is devoid of the pollution problem described above. Growing nanotubes uses their individual segments, carbon molecular implants and catalysts. The main disadvantage of cultivation is the complexity of this process and a small result.
There is another way to create nanotubes, which, at first glance, is devoid of drawbacks, is chemical vapor deposition with a floating catalyst (FC-CVD). Nanotubes can be produced in such a way quickly and in a large volume, and their properties will not be subject to negative changes. In addition, nanotubes can be assembled on a membrane filter to form thin films ready for use. All sounds very rosy, but here lies a tricky moment. Being in an aerosol medium, the catalysts can cause difficulties in the process of selective growth of nanotubes with low chirality. This problem can be solved by introducing a small amount of NH 3capable of narrowing the chiral distribution. However, N atoms can contaminate nanotubes at high temperatures, which will change its electronic properties.
What a way not to consider, there is always some unpleasant flaw, which has to be considered. However, researchers have proposed an option when it is possible to avoid the problems described above.
Sample creation and results
Scientists decided not to invent a new way to create nanotubes, but to improve the existing one, namely chemical deposition from the gas phase with a floating catalyst. The improvement method turned out to be quite simple - adding a small amount of CO 2 .
And now in order. Single-wall nanotubes were synthesized from CO (carbon source) at a volume flow rate of 350 cm 3 / min. Ferrocene ((η 5 –C 5 H 5 ) 2 Fe), carried by a flow of CO at 50 cm 3 / min, served as a catalyst .
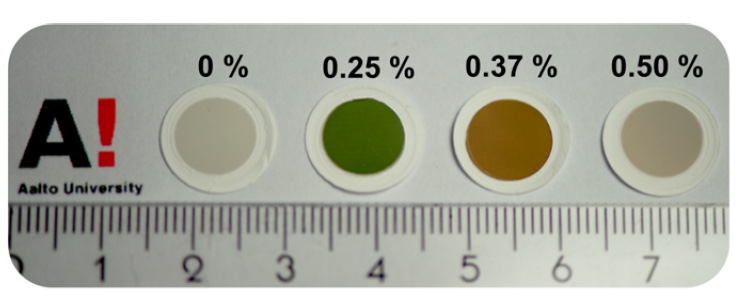
Setting up the process of growing nanotubes was carried out by introducing into the reactor a different volume of CO 2with a volume flow rate of 0, 1, 1.5 and 2.0 cm 3 / min, which corresponds to such volume fractions: 0, 0.25, 0.37 and 0.50 vol.%. The temperature at the same time was 850 or 880 ° C.
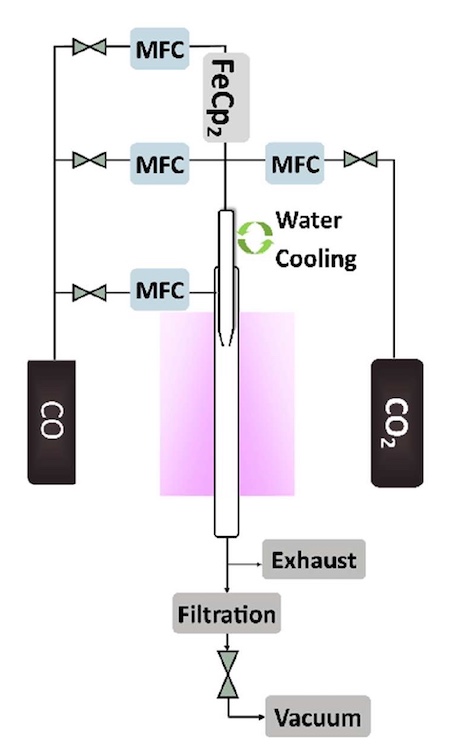
The scheme of the reactor operation
The introduction of a different volume of CO 2 led to the fact that the films of nanotubes turned out in different colors. This is clearly visible in the image below. These films were obtained at a temperature of 850 ° C.

After conducting transmission electron microscopy and energy dispersive X-ray spectroscopy, the researchers found that the color difference does not in any way affect the overall nanoparticle performance and size. It was also found that the samples have a high purity index.

Transmission electron microscopy (a, b, c) and dark field microscopy (d, e, f) of three samples with different volume fractions of CO 2 .
The average diameter of the nanotubes also depends directly on the concentration of CO 2 . So for 0, 0.25, 0.37 and 0.50 vol.% The average diameter was 1.1, 1.3, 1.8 and 1.9 nm, respectively.
Due to the fact that the color of the film and the diameter of the nanotubes reflect the concentration of CO 2 , it is logical to assume that this impurity in one way or another also changes the properties of the nanotubes.
The green sample (0.25 vol.%) Shows rather pronounced sharp changes in the absorption index at a wavelength of about 610 nm, and in the brown sample (0.37 vol.%) - at 760 nm.

Absorption spectrum of samples with different volume fractions of CO2 .
But other images (0 and 0.5 vol.%), In which such jumps were not observed, do not have a bright color, but remain gray.
In order to consider more closely the dependence of the chirality distribution (n, m) on the concentration of CO 2 , an electron diffraction analysis of the sample was carried out.
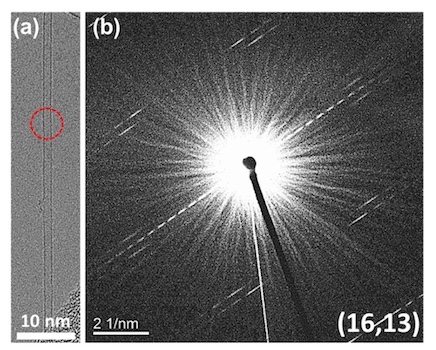
Electronic diffraction analysis
The image above ( a ) is a typical snapshot of a single-wall nanotube, and image b is the electron diffraction pattern (EDP) of this nanotube. After analyzing the line spacing, the chirality index was established - (16,13).

Electronic diffraction analysis of the sample 0 and 0.25 vol.%.
Carrying out the same analysis of working samples (images above) showed significantly better results: (8.7) and (11, 9).
As the CO 2 concentration increases, the diameter of the nanotubes also increases. With a volume fraction of CO 2 of 0.25 vol.%, The diameter is 1.0–1.5 nm. This indicator is directly related to the sample absorption rate.
It turns out that with the optimal diameter of the nanotube and a fairly good indicator of the distribution of chirality, the sample has a green color. Otherwise, we see a gray color. This remark should be correlated with the concentration of CO 2 , that is, its optimum vol.% Is 0.25.
Another indicator of the structure of a nanotube is the angle of chirality (the angle between the direction of folding and the direction in which adjacent hexagons have a common side).

To get the tube, that is, to twist the graphite plane, you need to cut the last one along dashed lines and roll it along the vector R.
All the samples under consideration (0, 0.25 and 0.50) showed quite satisfactory chirality angle - 20 ° -30 °.
An electron diffraction analysis was also carried out to verify the electronic properties of a bundle of nanotubes. As it turned out, all the tubes in the bundle had a different angle of chirality: 3.1 °, 18.9 °, 26.1 °.

Electronic diffraction analysis of a bundle of nanotubes.
An interesting fact was also found: with an increase in CO 2 concentrationfrom 0 to 0.50 the percentage of metallic nanotubes (meaning electrical conductivity) increased from 29.8 to 46.3%. However, when the concentration reached 1.23 vol.%, The quality of the nanotubes greatly decreased.
Temperature plays no less a role in the process of creating nanotubes. At higher temperatures, the rate of CO decomposition can be reduced (the bases of the nanotubes in this study). This will provide an opportunity to better control the synthesis process with the achievement of a lower index of chiral distribution.
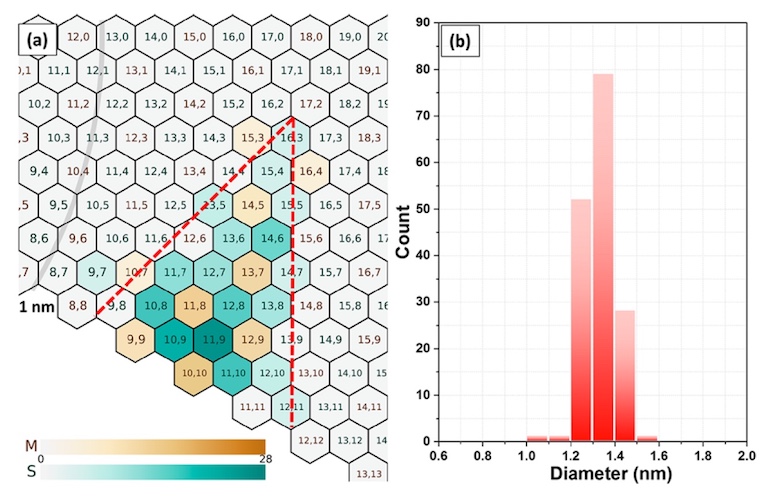
Variations of chirality (a) and diameter (b) of nanotubes at 0.25 vol.% CO 2 and a temperature of 880 ° C.
Comparing these figures with similar ones, but at a temperature of 850 ° C, it can be seen that chirality was obtained significantly lower, concentrated around (11.9). And the diameter of most tubes (more than 98%) varies in the range of 1.2-1.5 nm, which is an excellent result for this study.
Scientists report on their research is available here . And additional materials (graphs, pictures, tables, etc.) - here .
Epilogue
Scientists honestly say that much remains to be verified. For some indicators, such as conductivity and diameter, in samples without CO 2 and with CO 2 are not so impressively different as to be 100% sure of an unconditional victory. However, the importance of using CO 2 in the process of creating single-walled carbon nanotubes is definitely undeniable. This technique requires further study and refinement.
Among other things, scientists were able to successfully create nanotubes, the films of which differ in color due to differences in properties. Different concentration of CO 2changes the diameter of nanotubes and indicators of chirality, which as a result can give several color options for films: green, brown and gray. The color variety of such materials opens up new ways of their use, but changes will also occur in the existing ones.
This study is a vivid example of an extraordinary and innovative approach to solving the “old” issue and demonstrating to everyone the well-known truth “everything ingenious is simple”.
Thank you for staying with us. Do you like our articles? Want to see more interesting materials? Support us by placing an order or recommending to your friends, a 30% discount for Habr users for a unique analogue of the entry-level servers that we invented for you:The whole truth about VPS (KVM) E5-2650 v4 (6 Cores) 10GB DDR4 240GB SSD 1Gbps from $ 20 or how to share the server? (Options are available with RAID1 and RAID10, up to 24 cores and up to 40GB DDR4).
3 months for free if you pay for new Dell R630 for half a year - 2 x Intel Deca-Core Xeon E5-2630 v4 / 128GB DDR4 / 4x1TB HDD or 2x240GB SSD / 1Gbps 10 TB - from $ 99.33 a month , only until the end of August, order can be here .
Dell R730xd 2 times cheaper? Only we have 2 x Intel Dodeca-Core Xeon E5-2650v4 128GB DDR4 6x480GB SSD 1Gbps 100 TV from $ 249 in the Netherlands and the USA! Read about How to build an infrastructure building. class c using servers Dell R730xd E5-2650 v4 worth 9000 euros for a penny?
