Para- and magnetic resonances on the fingers and their application
Good day, Habr.
In this article I want to talk about a remarkable physical phenomenon as a magnetic moment relative to the center of mass. Where it comes from, how it can be imagined, and why every self-respecting ChemFac should use it.
It all started with the fact that the teacher of electricity and magnetism told us that a student who "caught" the EPR (electron paramagnetic resonance) using improvised means will get five automatically. Not for the sake of automaton, but for knowledge, only every second of my classmates decided to take up this matter, and together we collected a lot of information and I decided to share the most interesting.
To begin with, let us recall the structure of the atomic nucleus: it consists of nucleons - protons and neutrons fastening everything together. By the way, if you look at the periodic table, you can see that the number of neutrons grows faster than the number of protons (for comparison, helium has 2 protons and 2 neutrons, while uranium has 92 and 146, respectively), because protons are repelled (electromagnetic interaction) and more and more “cement” is needed to stabilize the nucleus (strong interaction), and very heavy elements decay themselves. Here in the form of a kernel and all business. The nucleus is far from always spherically symmetrical, and there is nothing to talk about molecules at all.
Now remember what a dipole is. A dipole is an electrically neutral, but extended (not a point) system. The simplest example is a system of two opposite but equal in absolute value charges located at a certain distance. By the way, the neutron itself is obtained when an electron falls on the nucleus and "merges" with the proton. They are looking for his dipole moment, but, alas, so far without success. But there will not be a dipole (it does not have a dipole moment) spherically symmetrically placed charges. With a magnetic dipole (the ancient name of systems with a magnetic moment) the same story. The formulas describing them have one form, but unlike a dipole (electric) it creates a similar magnetic field. It is also worth noting that the magnetic moment of the nucleus is composed of the spins of the nucleons of this nucleus.
Spin is the moment of anything relative to the center of mass. If we talk about "elementary particles", then in classical physics, the spin of, say, an electron, was presented as the rotation of this electron around an axis passing through the center of mass. But a closer look shows that this is not so because it turns out that the "equator" of the electron moves faster than the speed of light.
The magnetic moment is presented in the form of a vector and here an interesting picture is obtained: it turns out that if at a certain angle to the magnetic moment the lines of a constant magnetic field pass, then this vector itself will begin to rotate around an axis parallel to the magnetic lines.
And if you add a high-frequency alternating field, but rather weak relative to a constant, then for the three values: [the magnitude of the constant magnetic field (B), the cyclic frequency of the alternating magnetic field (w), some constant (G)], a correspondence is established at which resonance occurs.
w = G * B
In fact, a very intense absorption of the energy of the electromagnetic field begins, the magnetic moment, without ceasing to rotate (with the same frequency), becomes perpendicular to "B", and this is called resonance because of the similarity in the formulas.
All the charm in this “certain constant” is that it is different for each substance and each compound, that is, having one microwave generator (they are usually with a constant frequency or variable in a small range, others are difficult and not necessary), a constant magnetic field (about creating it later) with the possibility of changing its value, a waveguide from cardboard and foil and a detector (diode + ammeter ) you can determine the composition of almost any mixture. Almost any because not all atoms and molecules have a magnetic moment, but almost all. That is why every ChemFak is not ChemFak, if it does not have a setup for EPR and NMR. There are no fundamental differences between EPR (electron paramagnetic resonance) and NMR (nuclear magnetic resonance), the first uses the magnetic moment created by the electrons, and the other uses the nucleus.
Scheme of the simplest setup for EPR / NMR (I apologize for Paint):
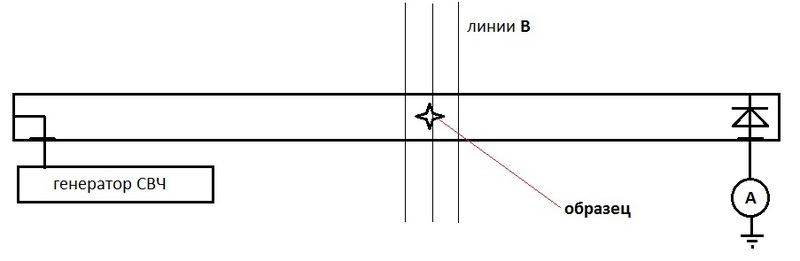
With resonant absorption of field energy by a sample, the ammeter / galvanometer will show zero.
Practical advice: It is
better to create a constant magnetic field with Helmholtz rings, since the formulas for calculating the magnetic field are simple, and the most difficult in their manufacture is the search for a thick wire. It is necessary to wind the foil with the mirror side inward (we also need it to reflect). The height and length of the square waveguide must be a multiple of half the wavelength.
Many thanks. I will be glad to talk about other phenomena in physics, write in the comments.
In this article I want to talk about a remarkable physical phenomenon as a magnetic moment relative to the center of mass. Where it comes from, how it can be imagined, and why every self-respecting ChemFac should use it.
It all started with the fact that the teacher of electricity and magnetism told us that a student who "caught" the EPR (electron paramagnetic resonance) using improvised means will get five automatically. Not for the sake of automaton, but for knowledge, only every second of my classmates decided to take up this matter, and together we collected a lot of information and I decided to share the most interesting.
To begin with, let us recall the structure of the atomic nucleus: it consists of nucleons - protons and neutrons fastening everything together. By the way, if you look at the periodic table, you can see that the number of neutrons grows faster than the number of protons (for comparison, helium has 2 protons and 2 neutrons, while uranium has 92 and 146, respectively), because protons are repelled (electromagnetic interaction) and more and more “cement” is needed to stabilize the nucleus (strong interaction), and very heavy elements decay themselves. Here in the form of a kernel and all business. The nucleus is far from always spherically symmetrical, and there is nothing to talk about molecules at all.
Now remember what a dipole is. A dipole is an electrically neutral, but extended (not a point) system. The simplest example is a system of two opposite but equal in absolute value charges located at a certain distance. By the way, the neutron itself is obtained when an electron falls on the nucleus and "merges" with the proton. They are looking for his dipole moment, but, alas, so far without success. But there will not be a dipole (it does not have a dipole moment) spherically symmetrically placed charges. With a magnetic dipole (the ancient name of systems with a magnetic moment) the same story. The formulas describing them have one form, but unlike a dipole (electric) it creates a similar magnetic field. It is also worth noting that the magnetic moment of the nucleus is composed of the spins of the nucleons of this nucleus.
Spin is the moment of anything relative to the center of mass. If we talk about "elementary particles", then in classical physics, the spin of, say, an electron, was presented as the rotation of this electron around an axis passing through the center of mass. But a closer look shows that this is not so because it turns out that the "equator" of the electron moves faster than the speed of light.
The magnetic moment is presented in the form of a vector and here an interesting picture is obtained: it turns out that if at a certain angle to the magnetic moment the lines of a constant magnetic field pass, then this vector itself will begin to rotate around an axis parallel to the magnetic lines.
And if you add a high-frequency alternating field, but rather weak relative to a constant, then for the three values: [the magnitude of the constant magnetic field (B), the cyclic frequency of the alternating magnetic field (w), some constant (G)], a correspondence is established at which resonance occurs.
w = G * B
In fact, a very intense absorption of the energy of the electromagnetic field begins, the magnetic moment, without ceasing to rotate (with the same frequency), becomes perpendicular to "B", and this is called resonance because of the similarity in the formulas.
All the charm in this “certain constant” is that it is different for each substance and each compound, that is, having one microwave generator (they are usually with a constant frequency or variable in a small range, others are difficult and not necessary), a constant magnetic field (about creating it later) with the possibility of changing its value, a waveguide from cardboard and foil and a detector (diode + ammeter ) you can determine the composition of almost any mixture. Almost any because not all atoms and molecules have a magnetic moment, but almost all. That is why every ChemFak is not ChemFak, if it does not have a setup for EPR and NMR. There are no fundamental differences between EPR (electron paramagnetic resonance) and NMR (nuclear magnetic resonance), the first uses the magnetic moment created by the electrons, and the other uses the nucleus.
Scheme of the simplest setup for EPR / NMR (I apologize for Paint):

With resonant absorption of field energy by a sample, the ammeter / galvanometer will show zero.
Practical advice: It is
better to create a constant magnetic field with Helmholtz rings, since the formulas for calculating the magnetic field are simple, and the most difficult in their manufacture is the search for a thick wire. It is necessary to wind the foil with the mirror side inward (we also need it to reflect). The height and length of the square waveguide must be a multiple of half the wavelength.
Many thanks. I will be glad to talk about other phenomena in physics, write in the comments.
