Evidences that have deceived scientists for several decades
- Transfer
In science, one can find surprisingly few proven facts. Instead, scientists often argue about how much evidence exists in favor of their theories. The more evidence, the stronger the theory and the more people agree with it.
Scientists are usually very careful in collecting evidence and scrutinizing their theories. But in the history of science there are several key, albeit rare, examples of how the evidence turned out to be so deceptive that they made the entire scientific community believe in what was later found completely wrong.
Usually, when collecting evidence, scientists make predictions of something, and see how right they are. Problems occur when the predictions are correct, and the theory used to create them is wrong. Predictions that seem particularly risky and turn out to be correct seem very convincing evidence, as Karl Popper and other philosophers of science often stressed . But history shows that even very strong evidence can deceive us.

Johann Friedrich Mekkel
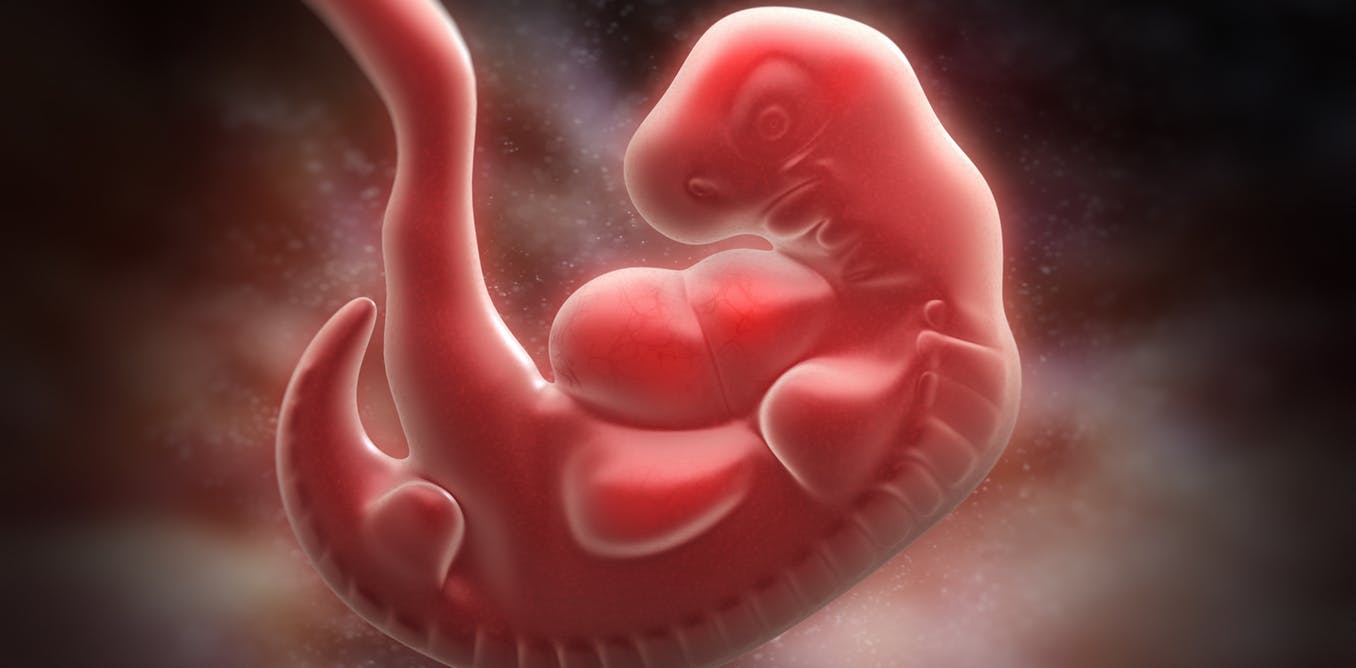
In 1811, Johann Friedrich Meckel successfully predicted that human embryos must have gill slits. This risky prediction was supposed to provide convincing evidence in favor of his theory that humans, as the “most advanced” organisms, evolve in stages corresponding to the “less ideal” species (fish, amphibians, reptiles, and so on).
Indeed, early human embryos have cracks in their necks resembling gills . And this is almost certainly due to the fact that humans and fish have a common DNA and a common ancestor, and not because we go through the “fish stage” while in the womb of mothers, as part of our development towards biological perfection.
But evidenceobtained after the discovery of cervical fissures in 1827, definitely added to the credibility of the theory of Meckel. Only when Charles Darwin’s theory of evolution made itself known in the second half of the 19th century did it become absolutely clear that Meckel’s idea of a linear sequence of biological perfection was completely unsuitable.

James Getton
Another example is the idea of a 18th century geologist James Getton [James Hutton; more accurate transcription - Hatton; His name is called the mineral huttonite / approx. trans.] that the Earth resembles an organic body, constantly reproducing itself in order to provide habitable environment for people. Based on this theory, Getton successfully predicted that granite veinsmust pass through other layers of stone and mix with it. He also successfully predicted inconsistent layers in which the new layers of stone rest at different angles to the older layers immediately below them.
The Getton theory was erroneous for a variety of reasons, compared with modern thinking. Obviously, the Earth is not made for humans. And, of course, Hetton had no idea about plate tectonics .
But, despite the theoretical errors, the predictions were successful, and became very influential. His theory remained a serious candidate for truth even 100 years later. And only at the end of the XIX century, it was finally supplanted by the contradictory theory of the Earthwhich (also mistakenly) explained the formation of lowlands and mountain ranges by the gradual compression of the Earth from cooling.
Mathematical evidence
The predictions of Meckel and Hetton were based on incorrect arguments. But there are some very impressive examples of deceptive evidence based on equations. For example, when Niels Bohr predicted in 1913 the correct frequencies for certain shades of light absorbed and emitted by ionized helium, Einstein, as they say, remarked : "So Bohr's theory must be correct."

Niels Bohr
Bohr’s predictions were able to instantly convince Einstein (and many others) because they were correct up to several decimal places. But they were taken from the model of the atom, in which electrons literally moved in circular orbits around atomic nuclei, which, as we now know, is not at all so. Boru was lucky: despite the fundamental fallacy of his model, it contained several grains of truth , enough to support his predictions about ionized helium.
But perhaps the most prominent example is related to the development of the Bohr model by Arnold Sommerfeld. Sommerfeld updated the model, giving the electron orbits an elliptical shape, and correcting it according to Einstein's theory of relativity. It all looked more realistic than the simple Bohr model.
Today we know that electrons do not actually move in orbits around nuclei. But scientists who worked at the beginning of the 20th century considered electrons to be tiny balls, and assumed that their movement can be compared with the movement of real balls.
This turned out to be a mistake: modern quantum mechanics tells us that electrons are terribly mysterious, and their behavior does not correspond to the usual concepts of man. Electrons in atoms do not even occupy an exact position at a certain time. All these arguments are described by a certain urgency: “If you think that you have understood quantum mechanics, then you have not understood it”.

Arnold Sommerfeld
So the erroneous view was at the very center of Sommerfeld's theory. And yet, in 1916, Sommerfeld used his model as the basis for an equation that correctly describes the subtle picture of the colors of light absorbed and emitted by hydrogen. This equation turned out to be exactly the same as what Paul Dirac derived.in 1928, using the modern theory of relativistic quantum mechanics.
This result in physical circles has long been considered a shocking coincidence, and there have been many attempts to understand how it turned out. Needless to say, the incredible predictive success of Sommerfeld’s theory convinced many scientists at the time of the truth of his theory.
Despite the fact that later evidence disproved these theories, I do not think we can say that the scientists involved in their development were wrong. They followed the testimony - that is how a good scientist should behave. They were not given to know that the evidence had led them astray.
These few examples clearly should not convince us that science can not be trusted. Testimonies are rarely completely erroneous, and usually completely false theories do not give out successful and accurate predictions (they usually give completely false predictions). Science is a process of continuous improvement, able to smooth out unnecessary imperfections in the long run. And we all know that even the most trustworthy things can sometimes let us down.
