Opus about His Majesty Clay. Part Four - Silicones
- Tutorial
Dedicated to Andrei A., whom I still have not apologized for the thing damaged by silicone ... We
continue our leisurelystories about fishing opus about adhesives. Our today's hero cannot boast of the title of a popular structural glue for any spaceships, but he is familiar with the absolute majority of readers. Because silicones are the main glues for all kinds of stickers, labels and adhesive tapes of the consumer (as opposed to professional adhesive tapes) segment. Well, to everything else, it is silicone that is most often used in sealing everything and everything. Under the cut - an explanation of why he can do it and how to help him do it even better. About "silicone - an inconspicuous worker."

RU Wikipedia tells us that silicones (polyorganosiloxanes, polysiloxanes) are oxygen-containing silicon polymers, the structure of which is represented by the main inorganic silicon-oxygen chain -Si-O-Si-O-Si-O- with attached organic side groups (they are attached to silicon atoms). Sometimes these lateral functional groups can join together two or more organosilicon chains, forming cross-links. By varying a) the length of the main organosilicon chain, b) lateral functional groups, and c) the number and type of cross bonds, silicones with different properties can be synthesized.

The first studies in the chemistry of silicones were carried out at the end of the 19th century by the English chemist Frederick Kipping (= "father of silicones") and it was he, by the way, who proposed the term silicone in 1901 . Thanks to this, many “translators” (mainly journalistic fraternities) still confuse silicones and silicon. Silicon is an inorganic material, a semiconductor, which is used for the manufacture of integrated circuits and solar cells. And silicones are organiccompounds that contain silicon, carbon, hydrogen, oxygen and sometimes other atoms, and are most often used as sealants, label adhesives and ... for example, implants for breast augmentation. So, I hope, the Habra reader will never confuse silicon (silicon) and silicone (silicone). Including when the conversation comes about " Silicon Valley ."
Moving away from historical parallels, we can say that the chemical businessmen, represented by Corning Glassworks (now Dow Corning) and General Electric, were the first to consider the commercial potential in silicones, and it was thanks to them that hundreds and thousands of various modifications, compositions, etc. were developed.
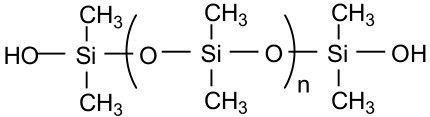
Most silicone sealants are based on silane-terminated polydimethylsiloxanes, which are often called silanols, for example: where n is usually 300 to 1600:
Due to their structure, these compounds occupy a unique intermediate position between inorganic and organic materials. The saturated inorganic Si-O-Si backbone provides excellent elasticity, flexibility and resistance to sunlight (mainly UV radiation), while organic functional groups are responsible for intermolecular forces. The following key features that determine the flexibility and durability of this class of materials can be noted:
- Low surface tension
- High water repellent ability
- Low glass transition temperature
- High gas permeability
- High thermal and oxidant resistance
- Insolubility in water
- High binding energy silicon-oxygen
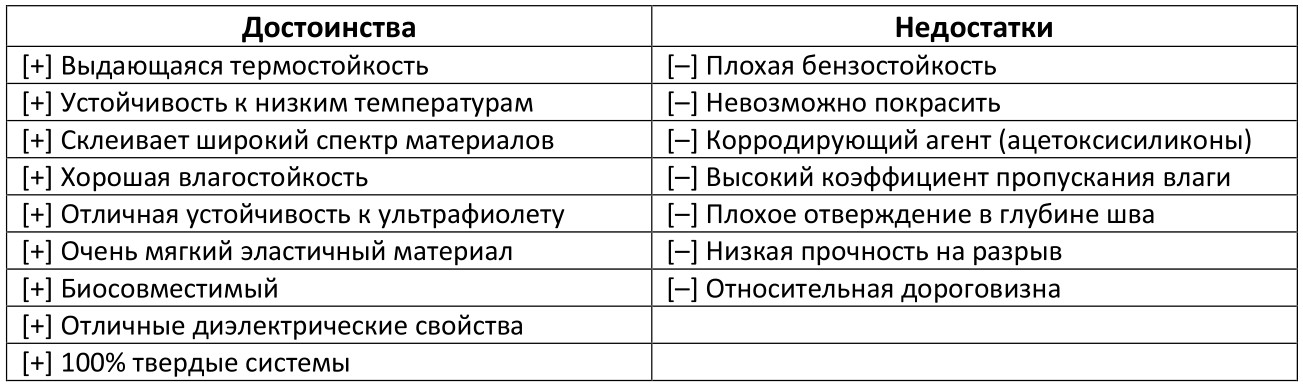
Silicone adhesives are not characterized by high strength (including peeling), they are mainly appreciated for their elasticity and ability to work in a wide temperature range (from cryotechnics from -115 ° C to more than 265 ° C with a plus sign, by the way, if need even higher, use supplements based on iron oxide). In simple terms, silicone adhesives are very sticky (so much so that due to low surface energy they can bind to polyethylene / polypropylene and fluorocarbons - hi sappience with a question about “ Duty Free bags ”).

In addition, low shrinkage and the ability to easily fill surface irregularities and gaps (due to low surface tension) make these materials ideal sealants in kitchens and bathrooms. Silicone is ideal when peel strength is more important than tensile or shear.
The low polarity of silicone elastomers gives these compounds excellent electrical insulating properties, which makes them highly resistant to high-voltage ionization and the formation of a corona discharge. Moreover, silicone adhesives and sealants retain their useful insulating properties at elevated temperatures due to the lack of thermal degradation.
The excellent resistance to ozone and ultraviolet radiation inherent in silicone polymers makes the use of specialized additives and additives unnecessary. Silicone elastomers can work for a long time without cracking and shrinkage in the open air in all climatic conditions. This is due to the fact that silicones are universally used for gluing glass when glazing buildings (that is, where significant values of thermal expansion of materials can be expected) or for the manufacture of tanks.
The high initial cost is more than compensated by high durability and the absence of the need to often carry out repairs.
Among the shortcomings can be noted, low resistance to gasoline, which somewhat limits the use of silicones in the car / aircraft industry. And the fact that this adhesive cures for a very long time in deep layers (it is not recommended to use a layer thicker than 12-13 mm) due to the characteristics of crosslinking by means of air humidity (= diffusion of moist air into the interior of the material slows down with increasing thickness of the adhesive layer, etc. and etc.). Low surface energy does not allow the paints to form good adhesion to the silicone coating (therefore, it is difficult to paint the sealant with ordinary paint), which is sometimes vital when you want to hide the glue line. And finally, depending on the type of silicone used (more on that below), acetic acid may be released during curing,
Silicone adhesives are divided into two main groups:
-Odnokomponentnye
-Dvuhkomponentnye
Single Componentsilicones contain polyorganosiloxane with -OH end groups and a crosslinking agent (e.g. methyl triacetoxysilane) that is sensitive to hydrolysis, that is, it reacts with water (when the material comes in contact with air moisture) as a result of a condensation reaction at room temperature. Therefore, often this type is also called RTV-1 (= vulcanization at room temperature, one-component). Acetic acid is a by-product of the condensation (using methyltriacetoxysilane as a hardener, although alcohol can also be released for the so-called neutral curing RTV-1). The cheapest, and therefore common (the basis of the vast majority of building and household sealants) silicone is acetoxysilicon.
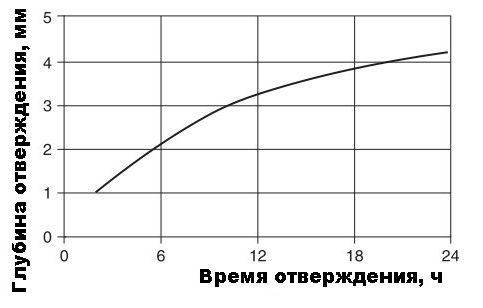
On average, curing is believed to occur at an average rate of approximately 2 mm per day. Since the process depends on air humidity, temperature and the thickness of the adhesive joint, it is logical to mention that to accelerate curing, it is necessary a) not to apply silicone with a very thick layer (6 mm maximum), b) to ensure maximum air contact of the adhesive joint with moist air (actively ventilate the room, do not use seams with complex geometry (spiral / broken, etc.), or seams inside objects without air access. Since acetic acid is released during the condensation process, the addition of components with an alkaline reaction (for example, alkaline means for cleaning pipes ala Mole or hydrated lime) will accelerate the curing process.The acid reacts with alkali, neutralizes and gives water, which in turn reacts with silicone (in addition to diffusion water taken from the air). Well, smelling like vinegar will obviously be weaker (by the way, if you need to wash off such silicone - “they kick out a wedge with a wedge” - do it most efficiently with acetic acid in a more concentrated way, at least 70% of the essence).
In addition to the unpleasant odor, as I mentioned, the released acetic acid can stimulate the processes of metal corrosion. For this reason, RTV-1 sealants using other hardeners have been developed.

Examples of the most commonly used compounds with their CAS numbers can be seen in the table below:
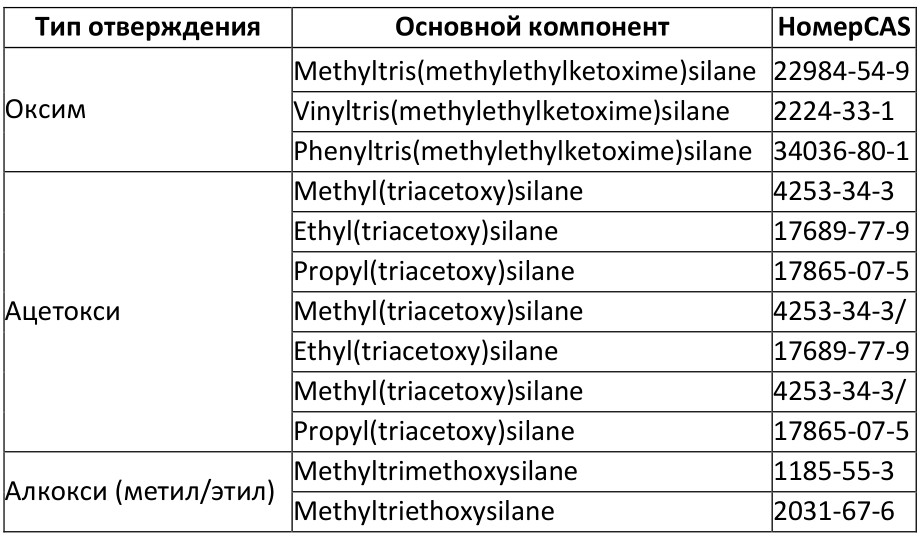
True, such silicones are a little more expensive, less common, and the curing process is slower. Comparison of different RTV-1s under the spoiler
If, for some reason, one-component silicones are not suitable, you can try two-component silicones , or RTV-2 (= vulcanization at room temperature, two-component). Such adhesives:
- cure much faster than the first group
- do not require high air humidity
- uniformly cure over the entire depth of the adhesive joint (regardless of width)
- do not emit dubious by-products during the curing process
A typical RTV-2 silicone consists of silanol + hardener with a catalyst. Organic tin compounds (tin octoate or dibutyltin dilaurate, which, by the way, will also work with “neutral” RTV-1 as curing catalysts) act as a catalyst. An increase in temperature speeds up the curing process. An example is the well-known silicone sealant SYLGARD 184 , which is actively used to seal solar modules.
Hardened, two-component silicones give slight shrinkage and provide better heat resistance among all silicone adhesives.
The performance characteristics of the silicone adhesive joint (RTV-2, although some points apply to RTV-1) are generally influenced by the following factors:
- Precursor structure
- Hardener dosage: too high a dose may make the adhesive joint too rigid, unstable, or generally uncured (see my ps)
- pH: pH accelerates the rate of hydrolysis and condensation.
- Many substances can act as catalysts: mineral acids, alkoxide salts, tin compounds, titanium esters, zirconium salts, phosphorus compounds and amines.
-System temperature: at elevated temperatures, the molecule moves faster / the number of collisions increases => curing is faster.
Under the spoiler is a table with well-known representatives of commercial silicones and their properties. We traditionally ask questions in the comments :)
Than "tear off the tiles":
Industrial washings:
- from “fathers of silicone” Dow Corning, DS-2025 for cured silicone - as a part of dodecylbenzenesulfonic acid (anionic surfactant), ABSK
- from “fathers of silicone” Dow Corning, DS-1000 for non-hardened silicone - as a part of nonionic surfactant - ethoxylated C6-C12 alcohol, quaternary ammonium compound , propylene glycol methyl ether (= solvent for washing nozzles of inkjet printer cartridges), (2-methoxymethylethoxy) propanol (washing liquid in printing).
PS My first acquaintance with two-component silicone
PPS All chemists with the past professional holiday! And chemical chemists - with the removal of “chemistry” from specialized hubs :) So shte, young people, we will now write articles in our free time.
Important! I can not call my articles in the full sense of the word popular science (“adult sciencepop”), since in most cases only a person with a fairly high scientific and technical background can read and digest what is written. In general, in the light of the latest “PAP-Habra-permutations,” while writing serial articles is suspended for an indefinite period.
If the community supports, we will continue . Going standard 5000 p. through “Support the author” - I am writing an article about epoxides . When translating, indicate the comment “continuation of articles on adhesives”. At the same time, we will check whether this topic is really interesting to the reader. 1950 rubles have already been collected .
Published earlier:
Opus about His Majesty Clay. Part One - Introductory
Opus about His Majesty Clay. Part Two - Viva, Cyanoacrylate! Viva, super glue
Opusa about His Majesty Clay. Part Three - Polyurethane vs Space Cold

continue our leisurely

RU Wikipedia tells us that silicones (polyorganosiloxanes, polysiloxanes) are oxygen-containing silicon polymers, the structure of which is represented by the main inorganic silicon-oxygen chain -Si-O-Si-O-Si-O- with attached organic side groups (they are attached to silicon atoms). Sometimes these lateral functional groups can join together two or more organosilicon chains, forming cross-links. By varying a) the length of the main organosilicon chain, b) lateral functional groups, and c) the number and type of cross bonds, silicones with different properties can be synthesized.

The first studies in the chemistry of silicones were carried out at the end of the 19th century by the English chemist Frederick Kipping (= "father of silicones") and it was he, by the way, who proposed the term silicone in 1901 . Thanks to this, many “translators” (mainly journalistic fraternities) still confuse silicones and silicon. Silicon is an inorganic material, a semiconductor, which is used for the manufacture of integrated circuits and solar cells. And silicones are organiccompounds that contain silicon, carbon, hydrogen, oxygen and sometimes other atoms, and are most often used as sealants, label adhesives and ... for example, implants for breast augmentation. So, I hope, the Habra reader will never confuse silicon (silicon) and silicone (silicone). Including when the conversation comes about " Silicon Valley ."
Moving away from historical parallels, we can say that the chemical businessmen, represented by Corning Glassworks (now Dow Corning) and General Electric, were the first to consider the commercial potential in silicones, and it was thanks to them that hundreds and thousands of various modifications, compositions, etc. were developed.
Most silicone sealants are based on silane-terminated polydimethylsiloxanes, which are often called silanols, for example: where n is usually 300 to 1600:

Due to their structure, these compounds occupy a unique intermediate position between inorganic and organic materials. The saturated inorganic Si-O-Si backbone provides excellent elasticity, flexibility and resistance to sunlight (mainly UV radiation), while organic functional groups are responsible for intermolecular forces. The following key features that determine the flexibility and durability of this class of materials can be noted:
- Low surface tension
- High water repellent ability
- Low glass transition temperature
- High gas permeability
- High thermal and oxidant resistance
- Insolubility in water
- High binding energy silicon-oxygen

Silicone adhesives are not characterized by high strength (including peeling), they are mainly appreciated for their elasticity and ability to work in a wide temperature range (from cryotechnics from -115 ° C to more than 265 ° C with a plus sign, by the way, if need even higher, use supplements based on iron oxide). In simple terms, silicone adhesives are very sticky (so much so that due to low surface energy they can bind to polyethylene / polypropylene and fluorocarbons - hi sappience with a question about “ Duty Free bags ”).

In addition, low shrinkage and the ability to easily fill surface irregularities and gaps (due to low surface tension) make these materials ideal sealants in kitchens and bathrooms. Silicone is ideal when peel strength is more important than tensile or shear.
Construction : installation and installation of glazing (wood, metals, plastics, glass), filling gaps and expansion joints on facades and masonry, waterproof seals when creating containers and an aquarium (industrial and domestic), seals in refrigeration and air conditioning systems.
The low polarity of silicone elastomers gives these compounds excellent electrical insulating properties, which makes them highly resistant to high-voltage ionization and the formation of a corona discharge. Moreover, silicone adhesives and sealants retain their useful insulating properties at elevated temperatures due to the lack of thermal degradation.
Electronics: low viscosity insulating silicones are used to fill electronic components (compounds). Metal-filled silicones are used as conductive adhesives.
The excellent resistance to ozone and ultraviolet radiation inherent in silicone polymers makes the use of specialized additives and additives unnecessary. Silicone elastomers can work for a long time without cracking and shrinkage in the open air in all climatic conditions. This is due to the fact that silicones are universally used for gluing glass when glazing buildings (that is, where significant values of thermal expansion of materials can be expected) or for the manufacture of tanks.

The high initial cost is more than compensated by high durability and the absence of the need to often carry out repairs.
Rocket science: silicones are used in high vacuum technology (including space), in the construction of nuclear power plants (in laboratory nuclear facilities, etc.) because of their radiation resistance.
Among the shortcomings can be noted, low resistance to gasoline, which somewhat limits the use of silicones in the car / aircraft industry. And the fact that this adhesive cures for a very long time in deep layers (it is not recommended to use a layer thicker than 12-13 mm) due to the characteristics of crosslinking by means of air humidity (= diffusion of moist air into the interior of the material slows down with increasing thickness of the adhesive layer, etc. and etc.). Low surface energy does not allow the paints to form good adhesion to the silicone coating (therefore, it is difficult to paint the sealant with ordinary paint), which is sometimes vital when you want to hide the glue line. And finally, depending on the type of silicone used (more on that below), acetic acid may be released during curing,
Varieties of Silicone Adhesives
Silicone adhesives are divided into two main groups:
-Odnokomponentnye
-Dvuhkomponentnye
Single Componentsilicones contain polyorganosiloxane with -OH end groups and a crosslinking agent (e.g. methyl triacetoxysilane) that is sensitive to hydrolysis, that is, it reacts with water (when the material comes in contact with air moisture) as a result of a condensation reaction at room temperature. Therefore, often this type is also called RTV-1 (= vulcanization at room temperature, one-component). Acetic acid is a by-product of the condensation (using methyltriacetoxysilane as a hardener, although alcohol can also be released for the so-called neutral curing RTV-1). The cheapest, and therefore common (the basis of the vast majority of building and household sealants) silicone is acetoxysilicon.

On average, curing is believed to occur at an average rate of approximately 2 mm per day. Since the process depends on air humidity, temperature and the thickness of the adhesive joint, it is logical to mention that to accelerate curing, it is necessary a) not to apply silicone with a very thick layer (6 mm maximum), b) to ensure maximum air contact of the adhesive joint with moist air (actively ventilate the room, do not use seams with complex geometry (spiral / broken, etc.), or seams inside objects without air access. Since acetic acid is released during the condensation process, the addition of components with an alkaline reaction (for example, alkaline means for cleaning pipes ala Mole or hydrated lime) will accelerate the curing process.The acid reacts with alkali, neutralizes and gives water, which in turn reacts with silicone (in addition to diffusion water taken from the air). Well, smelling like vinegar will obviously be weaker (by the way, if you need to wash off such silicone - “they kick out a wedge with a wedge” - do it most efficiently with acetic acid in a more concentrated way, at least 70% of the essence).
In addition to the unpleasant odor, as I mentioned, the released acetic acid can stimulate the processes of metal corrosion. For this reason, RTV-1 sealants using other hardeners have been developed.

Examples of the most commonly used compounds with their CAS numbers can be seen in the table below:

True, such silicones are a little more expensive, less common, and the curing process is slower. Comparison of different RTV-1s under the spoiler
Comparison of different types of RTV-1

If, for some reason, one-component silicones are not suitable, you can try two-component silicones , or RTV-2 (= vulcanization at room temperature, two-component). Such adhesives:
- cure much faster than the first group
- do not require high air humidity
- uniformly cure over the entire depth of the adhesive joint (regardless of width)
- do not emit dubious by-products during the curing process
A typical RTV-2 silicone consists of silanol + hardener with a catalyst. Organic tin compounds (tin octoate or dibutyltin dilaurate, which, by the way, will also work with “neutral” RTV-1 as curing catalysts) act as a catalyst. An increase in temperature speeds up the curing process. An example is the well-known silicone sealant SYLGARD 184 , which is actively used to seal solar modules.

Hardened, two-component silicones give slight shrinkage and provide better heat resistance among all silicone adhesives.
The performance characteristics of the silicone adhesive joint (RTV-2, although some points apply to RTV-1) are generally influenced by the following factors:
- Precursor structure
- Hardener dosage: too high a dose may make the adhesive joint too rigid, unstable, or generally uncured (see my ps)
- pH: pH accelerates the rate of hydrolysis and condensation.
- Many substances can act as catalysts: mineral acids, alkoxide salts, tin compounds, titanium esters, zirconium salts, phosphorus compounds and amines.
-System temperature: at elevated temperatures, the molecule moves faster / the number of collisions increases => curing is faster.
Under the spoiler is a table with well-known representatives of commercial silicones and their properties. We traditionally ask questions in the comments :)
Foreign commercial silicone adhesives
Table legend:
TS : tensile strength
SG : specific gravity (density relative to water density).
HS : Shore hardness (A or D)
EL (%) : tensile strength during tensile testing,%.
Visc. (Pa.s) : viscosity index (pascal second)
Service (° C) : operating temperature of the cured adhesive joint
Cure : type of cure to obtain standard joint strength. RT-room temperature (if there is a figure, this is the heating temperature).
Cure Rate (mm / day): cure rate (mm / day)
Skin Time (mins): setting time (minimum adhesive strength required)
Designations:
1n = one-component neutral, 1a = one-component acid, 2 - two-component
Circles indicate the properties of a particular adhesive with respect to all adhesives of this class: black circle - highest, black and white circle - moderate, white circle - weak.
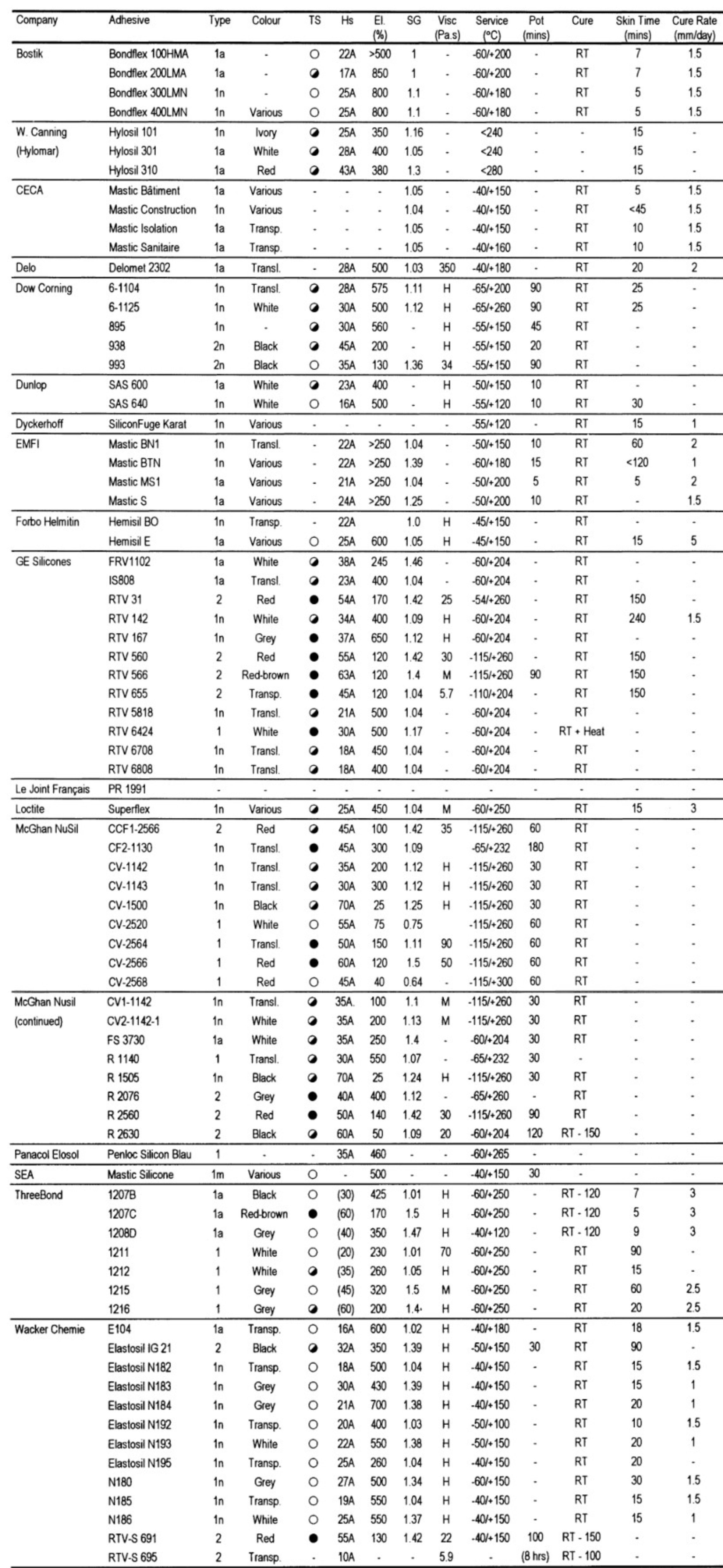
TS : tensile strength
SG : specific gravity (density relative to water density).
HS : Shore hardness (A or D)
EL (%) : tensile strength during tensile testing,%.
Visc. (Pa.s) : viscosity index (pascal second)
Service (° C) : operating temperature of the cured adhesive joint
Cure : type of cure to obtain standard joint strength. RT-room temperature (if there is a figure, this is the heating temperature).
Cure Rate (mm / day): cure rate (mm / day)
Skin Time (mins): setting time (minimum adhesive strength required)
Designations:
1n = one-component neutral, 1a = one-component acid, 2 - two-component
Circles indicate the properties of a particular adhesive with respect to all adhesives of this class: black circle - highest, black and white circle - moderate, white circle - weak.

Than "tear off the tiles":
By the way, if you need to wash off such silicone - “they kick out a wedge with a wedge” - do it most efficiently with acetic acid in a more concentrated way, at least 70% of the essence
Industrial washings:
- from “fathers of silicone” Dow Corning, DS-2025 for cured silicone - as a part of dodecylbenzenesulfonic acid (anionic surfactant), ABSK
- from “fathers of silicone” Dow Corning, DS-1000 for non-hardened silicone - as a part of nonionic surfactant - ethoxylated C6-C12 alcohol, quaternary ammonium compound , propylene glycol methyl ether (= solvent for washing nozzles of inkjet printer cartridges), (2-methoxymethylethoxy) propanol (washing liquid in printing).
PS My first acquaintance with two-component silicone
about the epigraph ...
My acquaintance with two-component silicone went rather sadly (see the article’s dedication). The same Sylgard 184 was begged from acquaintances from an adjacent research institute to create a replica of the diffraction grating (from an old Soviet device, such as MDR-2 1200 strokes / mm). From this:

Not feeling a trick, I mixed the components as my colleagues recommended (“normally sets”) and placed the grate on this layer of sufficiently liquid silicone. Joyful went home anticipating what beauty I will receive in the morning. But in the morning I was met by the same, not frozen silicone, which was spread all over the grate. My hair stood on end (inside I felt that it was not just a lattice) and I began to try various washes. The situation was complicated by the fact that it is impossible to rub the diffraction grating - all the engraving will be destroyed. Only drive off the solvent with a stream of nitrogen. What I just did not try, even the rarest solvents (after all, how, a person trusted me, and I, I actually destroyed someone else's thing). In impotence, I decided to harden silicone with temperature. He hardened and now has densely buried the idea of preserving the diffraction lines of the grating. But I decided to finish the job. After trying 30 pieces of different solvents, it was possible to liquefy the hard mass only with glacial acetic acid. And blow off gas from the grate. I didn’t have the courage to look under the microscope at what remained of the micro-furrow lattice engraved on an aluminum substrate on the glass ... So the lattice still lies :(. I'm sorry, Andrei, if you read ...

Not feeling a trick, I mixed the components as my colleagues recommended (“normally sets”) and placed the grate on this layer of sufficiently liquid silicone. Joyful went home anticipating what beauty I will receive in the morning. But in the morning I was met by the same, not frozen silicone, which was spread all over the grate. My hair stood on end (inside I felt that it was not just a lattice) and I began to try various washes. The situation was complicated by the fact that it is impossible to rub the diffraction grating - all the engraving will be destroyed. Only drive off the solvent with a stream of nitrogen. What I just did not try, even the rarest solvents (after all, how, a person trusted me, and I, I actually destroyed someone else's thing). In impotence, I decided to harden silicone with temperature. He hardened and now has densely buried the idea of preserving the diffraction lines of the grating. But I decided to finish the job. After trying 30 pieces of different solvents, it was possible to liquefy the hard mass only with glacial acetic acid. And blow off gas from the grate. I didn’t have the courage to look under the microscope at what remained of the micro-furrow lattice engraved on an aluminum substrate on the glass ... So the lattice still lies :(. I'm sorry, Andrei, if you read ...
PPS All chemists with the past professional holiday! And chemical chemists - with the removal of “chemistry” from specialized hubs :) So shte, young people, we will now write articles in our free time.
Important! I can not call my articles in the full sense of the word popular science (“adult sciencepop”), since in most cases only a person with a fairly high scientific and technical background can read and digest what is written. In general, in the light of the latest “PAP-Habra-permutations,” while writing serial articles is suspended for an indefinite period.
If the community supports, we will continue . Going standard 5000 p. through “Support the author” - I am writing an article about epoxides . When translating, indicate the comment “continuation of articles on adhesives”. At the same time, we will check whether this topic is really interesting to the reader. 1950 rubles have already been collected .
Published earlier:
Opus about His Majesty Clay. Part One - Introductory
Opus about His Majesty Clay. Part Two - Viva, Cyanoacrylate! Viva, super glue
Opusa about His Majesty Clay. Part Three - Polyurethane vs Space Cold

