New membrane separates oil and water with ease
Separation of water and gasoline
Scientists from the University of South Australia have developed a hydrophilic membrane coating for the separation of oil and water, which fundamentally improves the filter's properties and makes it easy to clean after use.
The more rubbish the inhabitants of the Earth throw out every second, the more pleasant it is to receive news about the invention of a new way to make the planet cleaner. For example, a project is in full swing to rid the oceans of plastic . By the way, here's a video on the topic: a whale asks fishermen to help him with a package stuck in his mouth:
No less terrible consequences are oil spilled over the surface of the water. Everyone remembers the terrifying accident in the Gulf of Mexico, where one of the largest oil and gas giants, the British company BP, produced oil. As a result of the accident, according to environmentalists , more than half a million birds died .
Scientists have already figured out how to reduce the surface of the oil slick . But after that, the oil film must still be removed from the surface of the water. To do this, several different methods are used - the separation of oil and water using centrifugal forces, filtration, adsorption, mechanical collection.
Existing membranes that separate water and oil are divided into two types. Some are hydrophobic and oleophilic, water repellent and oil-permeable. But such filters are rather quickly contaminated with oil, after which they must either be cleaned with harmful chemicals or use new ones.
Others, which are currently gaining more and more recognition, are oleophobic - in the state moistened with water they repel oil and freely pass water. Their disadvantage is the need for careful and thorough wetting before use. If oil gets on a dry part of the filter (and this can easily happen when fighting an oil stain), it will be covered with it and lose its useful properties, and it will need to be cleaned with special detergents.

Destruction of oil after containment
But Australian scientists have created a membrane from a metal mesh coated with a hydrophilic polymer based on phosphorylcholine . In particular, it is used in medicine for the manufacture of stents - scaffolds placed in the lumen of hollow organs (for example, vessels).
Phosphorylcholine molecules are zwitterions , or bipolar ions. They are electrically neutral, but in their structure there are parts that carry both negative and positive charges localized on non-neighboring atoms. Such molecules simply “adore” water - in biological systems, they create an aqueous layer on the outside of the cells that prevents their pollution.
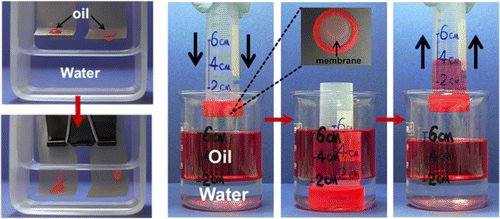
Its properties made it possible to create a membrane that makes it easy to remove a layer of oil from the surface of the water. To demonstrate its properties, the researchers made a test tube with such a membrane in the cap and immersed it in a glass of water, on the surface of which there was a 4-cm layer of oil. When immersed in oil, it freely penetrates the test tube, since the membrane was initially dry.

Separation of crude oil and sea water ( video insertion failed)
When the membrane collides with water, the water cleans the membrane of oil, it is wetted and acquires oleophobic properties. As a result, when lifting back all oil remains in a test tube, and water freely leaves.
According to scientists, such membranes can also be used as filters for industrial enterprises to prevent the release of oily liquids into the environment.
