Ask Ethan No. 79: The Smallest Neutron Star
- Transfer
What happens if a piece of a neutron star is broken off?

Sometimes the most interesting experiments in physics can be done only in your imagination. Despite the physical limitations that prevent us from going anywhere, cutting and studying in detail any object of the Universe that interests us, our understanding of matter - in all its manifestations - and the laws that govern it, has advanced quite far.
This week it was difficult for me to choose the most interesting question, but I settled on this brain-blowing question from Rui Carvalho, which sounds like this:
What kind of stars are these - neutron?

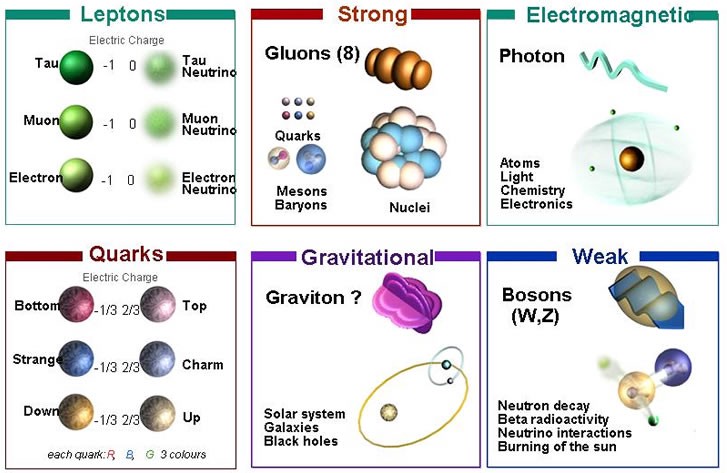
Judging by the name, these are balls of neutrons bound together due to the strongest gravitational attraction, with a mass approximately equal to the mass of a star like the Sun. But this, of course, is complete nonsense, since neutrons cannot exist for long. You can take any particle you are interested in, isolate and see what happens. For the three particles that make up most of normal matter — protons, neutrons, and electrons — the results will be very different.
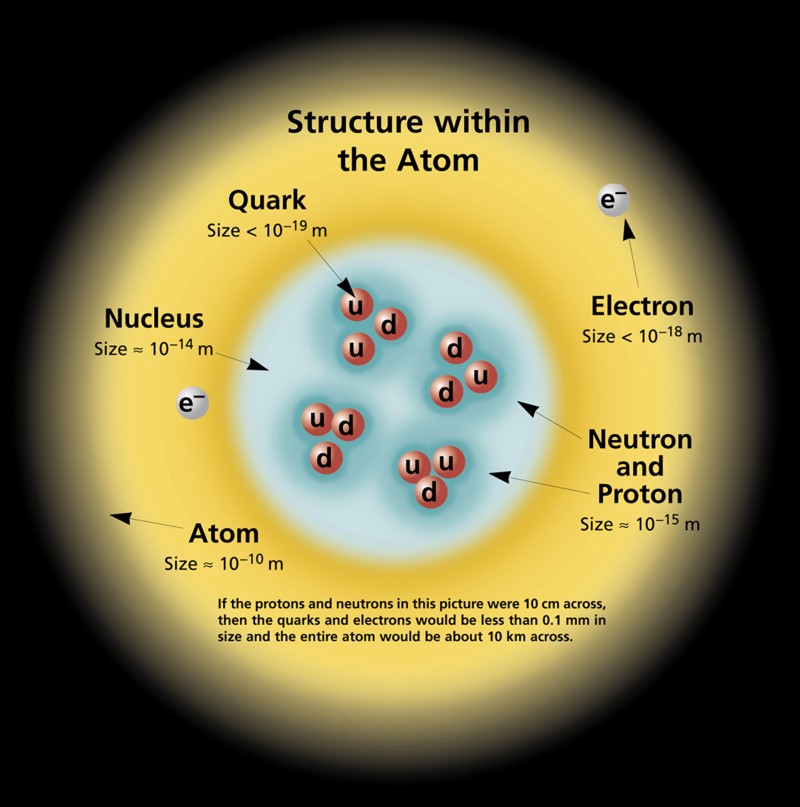
Electrons are the fundamental and easiest stable particles with an electric charge. As far as we know, they are absolutely stable and do not break up.
Protons are composite particles made up of quarks and gluons. In principle, they can break up and we tried to investigate this issue. We built huge containers filled with individual protons - each with 10 33 protons - and waited years to see if any of them decay. After decades, we found that even if the proton is unstable, its half-life is at least 10 35 years, or 10 25 times the age of the Universe. Apparently, they are also absolutely stable.
But not neutrons! If you observe a free neutron, it will most likely disappear after 15 minutes, decaying into a proton, electron and antineutrino (its half-life is 10 minutes).

And how then can we get such an object as a neutron star?
There is a difference between a free and a bound neutron, which is why many elements and isotopes do not decay: when a nucleus is bonded, it has a certain amount of binding energy sufficient to keep the neutrons stable.
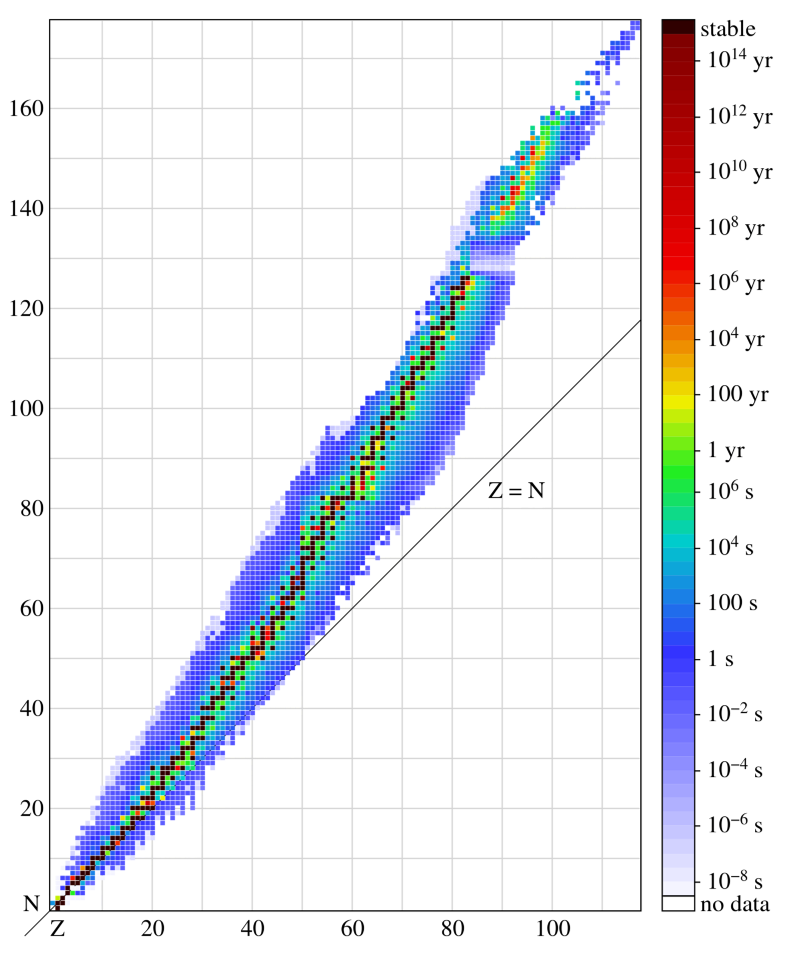
In the case of stable elements, some configurations turn out to be more stable than others, and there are approximately 254 stable variants in all. It is possible that on a large time scale some of them will turn out to be unstable - we just have not found this yet. But they are not particularly heavy and do not contain very many neutrons. The heaviest stable element is lead, number 82, with four known stable isotopes: Pb-204, Pb-206, Pb-207 and Pb-208.
So, of all the known stable elements, the heaviest is the nucleus with 82 protons and 126 neutrons.
But this is assuming that nuclear forces bind particles. And in the case of a neutron star, something else is responsible for this. To understand, let's understand how a neutron star arises.
The most massive stars - the brightest and bluest, born in young star clusters - synthesize helium from hydrogen in their nuclei, like all young stars. But unlike stars like the Sun, it takes them billions of years to burn all the fuel, not a few million, or even less, because ultra-high temperatures and densities lead to a very high synthesis rate.
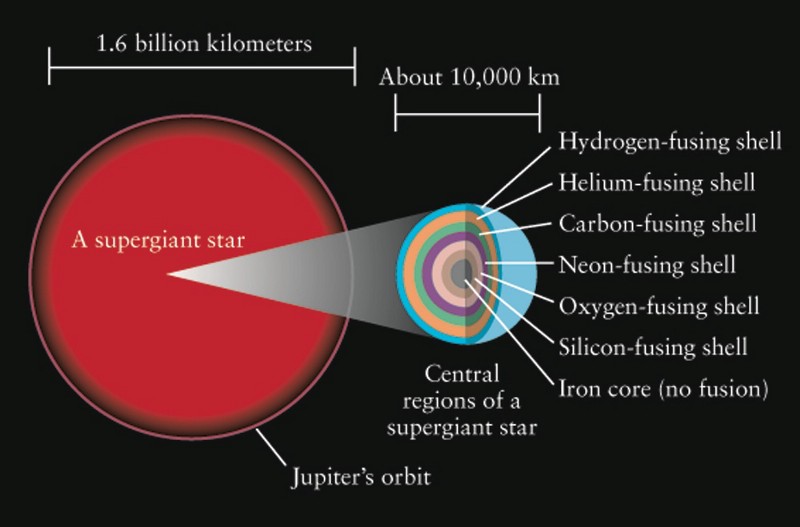
When they run out of hydrogen, the insides begin to shrink and warm up the star. Upon reaching the critical temperature in the star, carbon synthesis from helium begins, which leads to an even more active release of energy.
In just a few thousand years, both helium and the entrails are compressed even more, warming up to temperatures that the core of the sun will never reach. Under such extreme conditions, the synthesis of oxygen from carbon begins, and then, in subsequent reactions, silicon and sulfur are obtained from oxygen, iron from silicon, and then we have a problem.

Iron is the most stable element. With 26 protons and 30 neutrons, it receives the highest ratio of binding energy per nucleon, which means that all other combinations are less stable. (In some respects, nickel-62 is slightly more stable, but for simplicity we will focus on iron-56). Of course, there are elements that are heavier than iron, but they are not created by synthesis from iron. When the core is filled with iron, it begins to shrink under the influence of gravity, and there is no more fuel for combustion. And there remains an incredibly hot and dense plasma, which is constantly heated and compacted.
Upon reaching the critical value - surprise - the synthesis of electrons and protons begins, which leads to the appearance of neutrons, neutrinos and energy!

This reaction produces so much energy that in the supernova explosion the entire upper layer of the star is destroyed, since the synthesis of electrons and protons into neutrons and neutrinos takes only a few seconds.

The outer layers will take weeks and months to expand from the star, and the core is compressed to a ball of neutrons under the influence of a huge force, but not nuclear, but gravitational.
A neutron star weighs approximately like the Sun, and all this mass is concentrated in a volume with a radius of only a few kilometers. The density of the star is 10 19 kilograms per cubic meter and it is the densest three-dimensional object of the known in the universe.

In order for the neutron to be stable and not decay, its binding energy must exceed the mass difference of the neutron and proton, of the order of 1 MeV, or 0.1% of the neutron mass. The neutrons in the nucleus are quite simple to contain, and those on the surface will be in the most rarefied conditions. If the mass of a neutron star is equal to the solar one and the radius is 3 km, then the neutron bound on the surface will have 400 MeV of binding energy: enough to prevent its decay.
But what if we pull out a cubic centimeter of such matter, as Rui asks, from a neutron star? What do we get?

Unfortunately, the gravitational binding energy of neutrons on the surface will be only 0.07 eV - too little to keep them from decay!
In the real Universe, there are similar situations: when neutron stars collide with each other. Most of the matter merges and forms a black hole, but about 3% of the mass is thrown away. Instead of the formation of exotic matter, it quickly decays and leads to the appearance of a large part of the heaviest elements of the periodic table. If you were wondering where such elements as gold came from on Earth, it was from there: from the union of neutron stars!
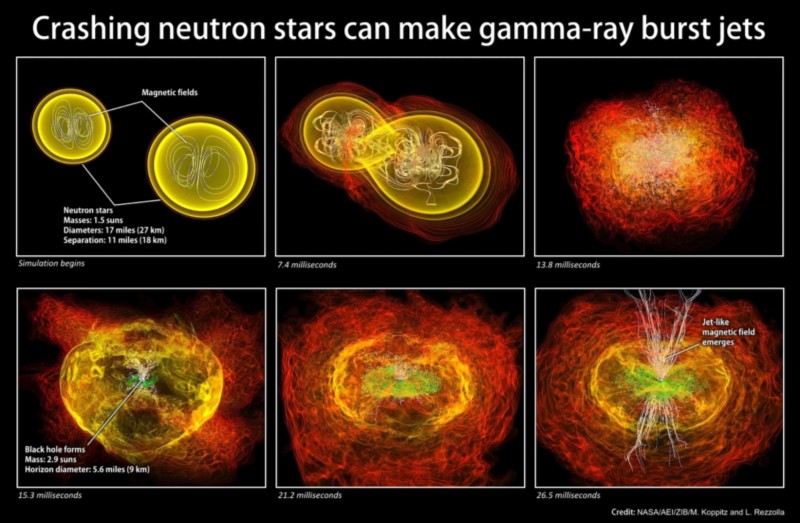
So, if you extract a small mass of neutrons, it will quickly decay into stable (or long-lived) elements and isotopes from the periodic table, in a time not exceeding the neutron lifetime, and most likely, much faster.
And if we wanted to break off a piece sufficient to ensure that neutrons remained stable on its surface? To do this, it must be a radius of at least 200 meters.
The mass of such a piece will be comparable to the mass of Saturn and this is only the lower limit on the mass necessary to achieve your goal. All that will have less mass will decay.
So, even if you wanted to believe that the hammer of Thor is made of the matter of a neutron star ...

Physics will not allow this. It is too small and the gravitational binding energy will be too small, as a result of which it will undergo rapid and catastrophic radioactive decay.
Thanks for the wonderful question, Rui, and I hope that if you dream of creating the smallest neutron star, you will start to think on a large scale!Send me your questions and suggestions for the following articles.

Imagine what it feels like to fall asleep and not wake up ... Now imagine how it feels to wake up if you haven’t fallen asleep.
- Alan Watts
Sometimes the most interesting experiments in physics can be done only in your imagination. Despite the physical limitations that prevent us from going anywhere, cutting and studying in detail any object of the Universe that interests us, our understanding of matter - in all its manifestations - and the laws that govern it, has advanced quite far.

This week it was difficult for me to choose the most interesting question, but I settled on this brain-blowing question from Rui Carvalho, which sounds like this:
If we broke off a piece of a neutron star (cubic centimeter) and removed it from it, what would happen to it?
What kind of stars are these - neutron?

Judging by the name, these are balls of neutrons bound together due to the strongest gravitational attraction, with a mass approximately equal to the mass of a star like the Sun. But this, of course, is complete nonsense, since neutrons cannot exist for long. You can take any particle you are interested in, isolate and see what happens. For the three particles that make up most of normal matter — protons, neutrons, and electrons — the results will be very different.

Electrons are the fundamental and easiest stable particles with an electric charge. As far as we know, they are absolutely stable and do not break up.
Protons are composite particles made up of quarks and gluons. In principle, they can break up and we tried to investigate this issue. We built huge containers filled with individual protons - each with 10 33 protons - and waited years to see if any of them decay. After decades, we found that even if the proton is unstable, its half-life is at least 10 35 years, or 10 25 times the age of the Universe. Apparently, they are also absolutely stable.
But not neutrons! If you observe a free neutron, it will most likely disappear after 15 minutes, decaying into a proton, electron and antineutrino (its half-life is 10 minutes).

And how then can we get such an object as a neutron star?
There is a difference between a free and a bound neutron, which is why many elements and isotopes do not decay: when a nucleus is bonded, it has a certain amount of binding energy sufficient to keep the neutrons stable.

In the case of stable elements, some configurations turn out to be more stable than others, and there are approximately 254 stable variants in all. It is possible that on a large time scale some of them will turn out to be unstable - we just have not found this yet. But they are not particularly heavy and do not contain very many neutrons. The heaviest stable element is lead, number 82, with four known stable isotopes: Pb-204, Pb-206, Pb-207 and Pb-208.
So, of all the known stable elements, the heaviest is the nucleus with 82 protons and 126 neutrons.

But this is assuming that nuclear forces bind particles. And in the case of a neutron star, something else is responsible for this. To understand, let's understand how a neutron star arises.
The most massive stars - the brightest and bluest, born in young star clusters - synthesize helium from hydrogen in their nuclei, like all young stars. But unlike stars like the Sun, it takes them billions of years to burn all the fuel, not a few million, or even less, because ultra-high temperatures and densities lead to a very high synthesis rate.
When they run out of hydrogen, the insides begin to shrink and warm up the star. Upon reaching the critical temperature in the star, carbon synthesis from helium begins, which leads to an even more active release of energy.
In just a few thousand years, both helium and the entrails are compressed even more, warming up to temperatures that the core of the sun will never reach. Under such extreme conditions, the synthesis of oxygen from carbon begins, and then, in subsequent reactions, silicon and sulfur are obtained from oxygen, iron from silicon, and then we have a problem.

Iron is the most stable element. With 26 protons and 30 neutrons, it receives the highest ratio of binding energy per nucleon, which means that all other combinations are less stable. (In some respects, nickel-62 is slightly more stable, but for simplicity we will focus on iron-56). Of course, there are elements that are heavier than iron, but they are not created by synthesis from iron. When the core is filled with iron, it begins to shrink under the influence of gravity, and there is no more fuel for combustion. And there remains an incredibly hot and dense plasma, which is constantly heated and compacted.
Upon reaching the critical value - surprise - the synthesis of electrons and protons begins, which leads to the appearance of neutrons, neutrinos and energy!

This reaction produces so much energy that in the supernova explosion the entire upper layer of the star is destroyed, since the synthesis of electrons and protons into neutrons and neutrinos takes only a few seconds.

The outer layers will take weeks and months to expand from the star, and the core is compressed to a ball of neutrons under the influence of a huge force, but not nuclear, but gravitational.
A neutron star weighs approximately like the Sun, and all this mass is concentrated in a volume with a radius of only a few kilometers. The density of the star is 10 19 kilograms per cubic meter and it is the densest three-dimensional object of the known in the universe.

In order for the neutron to be stable and not decay, its binding energy must exceed the mass difference of the neutron and proton, of the order of 1 MeV, or 0.1% of the neutron mass. The neutrons in the nucleus are quite simple to contain, and those on the surface will be in the most rarefied conditions. If the mass of a neutron star is equal to the solar one and the radius is 3 km, then the neutron bound on the surface will have 400 MeV of binding energy: enough to prevent its decay.
But what if we pull out a cubic centimeter of such matter, as Rui asks, from a neutron star? What do we get?

Unfortunately, the gravitational binding energy of neutrons on the surface will be only 0.07 eV - too little to keep them from decay!
In the real Universe, there are similar situations: when neutron stars collide with each other. Most of the matter merges and forms a black hole, but about 3% of the mass is thrown away. Instead of the formation of exotic matter, it quickly decays and leads to the appearance of a large part of the heaviest elements of the periodic table. If you were wondering where such elements as gold came from on Earth, it was from there: from the union of neutron stars!

So, if you extract a small mass of neutrons, it will quickly decay into stable (or long-lived) elements and isotopes from the periodic table, in a time not exceeding the neutron lifetime, and most likely, much faster.
And if we wanted to break off a piece sufficient to ensure that neutrons remained stable on its surface? To do this, it must be a radius of at least 200 meters.
The mass of such a piece will be comparable to the mass of Saturn and this is only the lower limit on the mass necessary to achieve your goal. All that will have less mass will decay.
So, even if you wanted to believe that the hammer of Thor is made of the matter of a neutron star ...


Physics will not allow this. It is too small and the gravitational binding energy will be too small, as a result of which it will undergo rapid and catastrophic radioactive decay.
Thanks for the wonderful question, Rui, and I hope that if you dream of creating the smallest neutron star, you will start to think on a large scale!Send me your questions and suggestions for the following articles.
