SENS-Diagnosis. Biomarkers of protein aggregates
Proteins play an important role in all living organisms, performing many different functions. As you know, they are composed of amino acids. In order to perform their functions, proteins must be not just a chain of certain amino acids, but have a certain spatial form, that is, they must be properly fit in space. For various reasons, a malfunction may occur in the normal folding of the protein into the desired structure. Then, instead of incorrectly folded proteins, which tend to combine into clusters, aggregates of proteins are amyloid fibrils. The most famous of these aggregates is β-amyloid (Aβ, Abeta), presumably associated with the development of neuropathology, as well as some types of cancer and one of the causes of dementia in individuals suffering from Down syndrome.
Such protein structures have a diameter of about 5–10 nm and a length of up to 800 nm, and consist of two or more parallel multidirectional filaments that form a specific structure — a cross-beta-folded conformation. It is this structure that determines the specific optical property of amyloid - the ability for birefringence. And the discovery of this property is the basis for the diagnosis of amyloidosis. Microscopy of Congo-stained dyes with polarized light causes amyloid to change its red color to a green glow [1].
Accumulation of abnormal proteins, the authors of the SENS concept are called “Extracellular junk” and define one of the causes of aging, which seems to be quite fair. Amyloid fibrils, by virtue of their structure, are not subject to the action of special enzymes that break down proteins (proteases), and therefore tend to accumulate in the tissues of the body, disrupting their work. The structural and chemical-physical properties of amyloid depend on the basic precursor protein, whose content in the fibril is about 80%, and this determines the specific trait for each type of amyloidosis. The term amyloidosis refers to a group of hereditary or acquired diseases associated with extracellular fibril deposition of insoluble proteins that cause tissue structural disorders and organ dysfunction. Currently, more than 20 amyloidogenic progenitor proteins and as many clinical variants of amyloidosis are known. In addition to the well-known β-amyloid, there is AA-amyloid associated with rheumatoid arthritis, heart disease, kidney disease and intestinal inflammation, AIAPP-amyloid, involved in the pathogenesis of type 2 diabetes, and others [2].
β-amyloid.
SENS see the use of specialized catalytically active antibodies, so-called abzymes (eng. Abzyme, antibody enzyme), which are chosen specifically for amyloids and remove them from the tissue, as a way to solve the problem of accumulation of protein aggregates. Recently, a promising approach was developed under this approach. A subset of human antibodies has been found that has catalytic activity against a particular antigen, breaking it up into smaller and less harmful fragments, rather than capturing it for removal or destruction by other immune cells. The use of these new catalytic antibodies as a therapy for targeting amyloids has potential advantages over sequestering antibodies used in other amyloid vaccines. The first is that a reduction in the dose needed to effectively remove extracellular aggregates from tissues is expected. This is due to the fact that sequestering antibodies can capture and then transport only one amyloid molecule at a time. Whereas abzymes bind to an amyloid molecule, crush it and then go on to the next one, one after another, allowing each antibody molecule to quickly destroy several amyloid molecules. Another is that catalytic antibodies belong to a class that is more efficiently transferred through the blood-brain barrier that protects our brain, while sequestering antibodies find it harder to overcome this barrier [3]. and then transport only one amyloid molecule at a time. Whereas abzymes bind to an amyloid molecule, crush it and then go on to the next one, one after another, allowing each antibody molecule to quickly destroy several amyloid molecules. Another is that catalytic antibodies belong to a class that is more efficiently transferred through the blood-brain barrier that protects our brain, while sequestering antibodies find it harder to overcome this barrier [3]. and then transport only one amyloid molecule at a time. Whereas abzymes bind to an amyloid molecule, crush it and then go on to the next one, one after another, allowing each antibody molecule to quickly destroy several amyloid molecules. Another is that catalytic antibodies belong to a class that is more efficiently transferred through the blood-brain barrier that protects our brain, while sequestering antibodies find it harder to overcome this barrier [3].
It is known that insoluble plaques in the brain of patients are formed mainly by β-amyloid peptide (Aβ), which has a molecular mass of 4 kDa and a length of about 40 amino acid residues. Aβ is a fragment of the transmembrane protein precursor beta amyloid (amyloid precursor protein, APP), which is found in many tissues of the body, including neuron synapses. APP is involved in some physiological processes associated with neuroplasticity, synapse formation and neuroprotection (survival of nerve cells) [4].
Unlike its predecessor, Aβ is toxic to nerve cells, contributing to their degeneration and death. It is formed by separating the extracellular N-terminal domain (sAPP) of the precursor protein. This process can be carried out by two different secretases - α-secretase and β-secretase, which have a fundamental difference in their action. In the first case, fragmentation occurs between amino acid residues within the Aβ sequence, which prevents the subsequent formation of the amyloid peptide. Secondly, pathological, under the influence of β-secretase, the process of fragmentation, due to its peculiarities, ends with the formation of Aβ. This second pathway, associated with the development of neuropathologies, is more rare, and why the fragmentation of the precursor protein goes along it remains not completely clear [5].
β-secretase.
β-amyloid oxidizes cholesterol and polyunsaturated fatty acids, forming the most toxic forms of the active forms of oxygen - hydroxyl radical and hydrogen peroxide. Racemization of L-aspartic acid in long-lived proteins contributes to the formation of β-amyloid and α-synuclein. Pathological accumulations of the latter are observed in Parkinson's disease, Alzheimer's disease, Levy's disease and other neurodegenerative diseases.
Another pathologic sign of neurodegeneration in Alzheimer's disease are aggregates of hyperphosphorylated tau protein (τ protein): paired helical filaments (PHF) and neurofibrillary tangles (NFT). Physiologically, the τ-protein is involved in the stabilization of microtubules of neurons, which ensure the transfer of cellular organelles, glycoproteins and other substances through the cytoplasm of neurons. In Alzheimer's disease, τ-protein is hyperphosphorylated, loses its normal ability to stabilize microtubules and accumulates in the cell by insoluble neurotoxic structures. What happens during the formation of pathology earlier - the formation of amyloid aggregates or τ-protein - the question is not completely clear. But it is obvious that these two processes are interrelated and stimulate each other. So, both pathological proteins exhibit prion properties, similar to the prion protein PrPsc: improperly curtailed forms of chain-reaction proteins stimulate the transformation of normal proteins into abnormal ones in the healthy neurons surrounding them. Between themselves, Aβ and τ-protein also interact by the type of prions: it is described how Aβ activates the protein kinase GSK3, phosphorylating the τ-protein and causing its improper fording [5].
τ-protein.
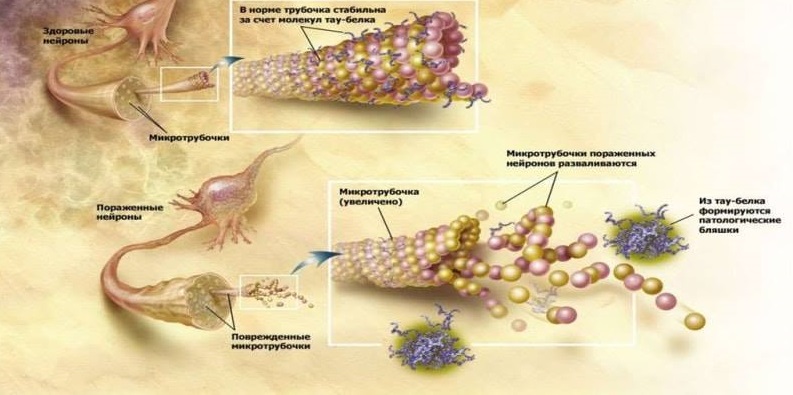
The functions of τ-protein are normal and pathological.
Another infection in which laboratory mice with elevated amyloidogenesis and normal mice were surgically pooled can confirm the infectivity of Alzheimer's disease. As a result, healthy mice began to accumulate Aβ in the brain, which is generally not characteristic of these rodents. This study for the first time showed the potential for penetration of Aβ with blood into the brain and subsequent participation in the development of neurodegeneration [10].
The relationship between the accumulation of β-amyloid and τ-protein in age-related neuropathology determines the need for finding effective methods to identify these protein aggregates as biomarkers of future neuropathology and accelerated aging. Today several such methods are already known.
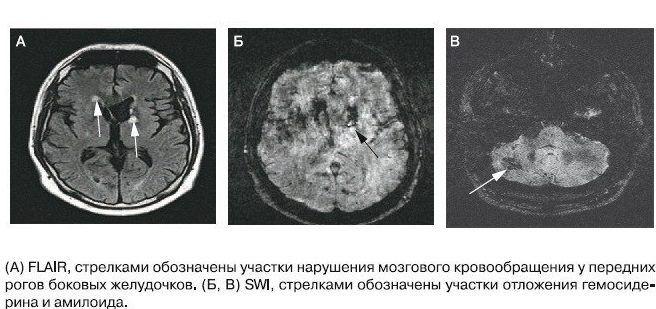
One of the most sensitive biomarkers in the early diagnosis of Alzheimer's disease and moderate congenital disorders (CCN) in the pathological phase is today the levels of amyloid Aβ-42, total τ-protein and phosphorylated τ-protein in the cerebrospinal fluid (CSF). Moreover, in pathology, amyloids show a decrease in the level, and the levels in the cerebrospinal fluid τ-protein, total and phosphorylated - increase. This is due to the fact that healthy people do not have accumulations of amyloid in the form of plaques and, therefore, a large amount of free amyloid is found in the liquor. Low τ-protein values show the absence of destruction of the neuronal cytoskeleton.
A significant correlation was found between age and slowing down the excretion of β-amyloid from the nervous system, which may be associated with sclerosis of the lymphatic vessels and a decrease in their drainage abilities. Restoration of drainage is one of the most promising methods of treatment of Alzheimer's disease, proposed by Leucadia, an interview with the founder of which was translated and published on habr.com/post/371513
Ultrasensitive immunological and mass spectrometric methods for diagnosing amyloidosis (Aβ42 / 40 or APP669-711 / Aβ42 proteins) and neurodegeneration ratios (τ proteins and neurofilaments) are expected to appear in plasma [12]. There are also “markless” methods for detecting incorrectly folded proteins in plasma [13].
Review authors: Denis Odinokov, Alexey Rzheshevsky.
Such protein structures have a diameter of about 5–10 nm and a length of up to 800 nm, and consist of two or more parallel multidirectional filaments that form a specific structure — a cross-beta-folded conformation. It is this structure that determines the specific optical property of amyloid - the ability for birefringence. And the discovery of this property is the basis for the diagnosis of amyloidosis. Microscopy of Congo-stained dyes with polarized light causes amyloid to change its red color to a green glow [1].
Accumulation of abnormal proteins, the authors of the SENS concept are called “Extracellular junk” and define one of the causes of aging, which seems to be quite fair. Amyloid fibrils, by virtue of their structure, are not subject to the action of special enzymes that break down proteins (proteases), and therefore tend to accumulate in the tissues of the body, disrupting their work. The structural and chemical-physical properties of amyloid depend on the basic precursor protein, whose content in the fibril is about 80%, and this determines the specific trait for each type of amyloidosis. The term amyloidosis refers to a group of hereditary or acquired diseases associated with extracellular fibril deposition of insoluble proteins that cause tissue structural disorders and organ dysfunction. Currently, more than 20 amyloidogenic progenitor proteins and as many clinical variants of amyloidosis are known. In addition to the well-known β-amyloid, there is AA-amyloid associated with rheumatoid arthritis, heart disease, kidney disease and intestinal inflammation, AIAPP-amyloid, involved in the pathogenesis of type 2 diabetes, and others [2].

β-amyloid.
SENS see the use of specialized catalytically active antibodies, so-called abzymes (eng. Abzyme, antibody enzyme), which are chosen specifically for amyloids and remove them from the tissue, as a way to solve the problem of accumulation of protein aggregates. Recently, a promising approach was developed under this approach. A subset of human antibodies has been found that has catalytic activity against a particular antigen, breaking it up into smaller and less harmful fragments, rather than capturing it for removal or destruction by other immune cells. The use of these new catalytic antibodies as a therapy for targeting amyloids has potential advantages over sequestering antibodies used in other amyloid vaccines. The first is that a reduction in the dose needed to effectively remove extracellular aggregates from tissues is expected. This is due to the fact that sequestering antibodies can capture and then transport only one amyloid molecule at a time. Whereas abzymes bind to an amyloid molecule, crush it and then go on to the next one, one after another, allowing each antibody molecule to quickly destroy several amyloid molecules. Another is that catalytic antibodies belong to a class that is more efficiently transferred through the blood-brain barrier that protects our brain, while sequestering antibodies find it harder to overcome this barrier [3]. and then transport only one amyloid molecule at a time. Whereas abzymes bind to an amyloid molecule, crush it and then go on to the next one, one after another, allowing each antibody molecule to quickly destroy several amyloid molecules. Another is that catalytic antibodies belong to a class that is more efficiently transferred through the blood-brain barrier that protects our brain, while sequestering antibodies find it harder to overcome this barrier [3]. and then transport only one amyloid molecule at a time. Whereas abzymes bind to an amyloid molecule, crush it and then go on to the next one, one after another, allowing each antibody molecule to quickly destroy several amyloid molecules. Another is that catalytic antibodies belong to a class that is more efficiently transferred through the blood-brain barrier that protects our brain, while sequestering antibodies find it harder to overcome this barrier [3].
It is known that insoluble plaques in the brain of patients are formed mainly by β-amyloid peptide (Aβ), which has a molecular mass of 4 kDa and a length of about 40 amino acid residues. Aβ is a fragment of the transmembrane protein precursor beta amyloid (amyloid precursor protein, APP), which is found in many tissues of the body, including neuron synapses. APP is involved in some physiological processes associated with neuroplasticity, synapse formation and neuroprotection (survival of nerve cells) [4].
Unlike its predecessor, Aβ is toxic to nerve cells, contributing to their degeneration and death. It is formed by separating the extracellular N-terminal domain (sAPP) of the precursor protein. This process can be carried out by two different secretases - α-secretase and β-secretase, which have a fundamental difference in their action. In the first case, fragmentation occurs between amino acid residues within the Aβ sequence, which prevents the subsequent formation of the amyloid peptide. Secondly, pathological, under the influence of β-secretase, the process of fragmentation, due to its peculiarities, ends with the formation of Aβ. This second pathway, associated with the development of neuropathologies, is more rare, and why the fragmentation of the precursor protein goes along it remains not completely clear [5].

β-secretase.
β-amyloid oxidizes cholesterol and polyunsaturated fatty acids, forming the most toxic forms of the active forms of oxygen - hydroxyl radical and hydrogen peroxide. Racemization of L-aspartic acid in long-lived proteins contributes to the formation of β-amyloid and α-synuclein. Pathological accumulations of the latter are observed in Parkinson's disease, Alzheimer's disease, Levy's disease and other neurodegenerative diseases.
Another pathologic sign of neurodegeneration in Alzheimer's disease are aggregates of hyperphosphorylated tau protein (τ protein): paired helical filaments (PHF) and neurofibrillary tangles (NFT). Physiologically, the τ-protein is involved in the stabilization of microtubules of neurons, which ensure the transfer of cellular organelles, glycoproteins and other substances through the cytoplasm of neurons. In Alzheimer's disease, τ-protein is hyperphosphorylated, loses its normal ability to stabilize microtubules and accumulates in the cell by insoluble neurotoxic structures. What happens during the formation of pathology earlier - the formation of amyloid aggregates or τ-protein - the question is not completely clear. But it is obvious that these two processes are interrelated and stimulate each other. So, both pathological proteins exhibit prion properties, similar to the prion protein PrPsc: improperly curtailed forms of chain-reaction proteins stimulate the transformation of normal proteins into abnormal ones in the healthy neurons surrounding them. Between themselves, Aβ and τ-protein also interact by the type of prions: it is described how Aβ activates the protein kinase GSK3, phosphorylating the τ-protein and causing its improper fording [5].

τ-protein.

The functions of τ-protein are normal and pathological.
Another infection in which laboratory mice with elevated amyloidogenesis and normal mice were surgically pooled can confirm the infectivity of Alzheimer's disease. As a result, healthy mice began to accumulate Aβ in the brain, which is generally not characteristic of these rodents. This study for the first time showed the potential for penetration of Aβ with blood into the brain and subsequent participation in the development of neurodegeneration [10].
The relationship between the accumulation of β-amyloid and τ-protein in age-related neuropathology determines the need for finding effective methods to identify these protein aggregates as biomarkers of future neuropathology and accelerated aging. Today several such methods are already known.

One of the most sensitive biomarkers in the early diagnosis of Alzheimer's disease and moderate congenital disorders (CCN) in the pathological phase is today the levels of amyloid Aβ-42, total τ-protein and phosphorylated τ-protein in the cerebrospinal fluid (CSF). Moreover, in pathology, amyloids show a decrease in the level, and the levels in the cerebrospinal fluid τ-protein, total and phosphorylated - increase. This is due to the fact that healthy people do not have accumulations of amyloid in the form of plaques and, therefore, a large amount of free amyloid is found in the liquor. Low τ-protein values show the absence of destruction of the neuronal cytoskeleton.
A significant correlation was found between age and slowing down the excretion of β-amyloid from the nervous system, which may be associated with sclerosis of the lymphatic vessels and a decrease in their drainage abilities. Restoration of drainage is one of the most promising methods of treatment of Alzheimer's disease, proposed by Leucadia, an interview with the founder of which was translated and published on habr.com/post/371513
Ultrasensitive immunological and mass spectrometric methods for diagnosing amyloidosis (Aβ42 / 40 or APP669-711 / Aβ42 proteins) and neurodegeneration ratios (τ proteins and neurofilaments) are expected to appear in plasma [12]. There are also “markless” methods for detecting incorrectly folded proteins in plasma [13].
Review authors: Denis Odinokov, Alexey Rzheshevsky.
Bibliography
- Rameev V., Kozlovskaya L. Amyloidosis: modern methods of diagnosis and treatment. Effective pharmacotherapy. Urology and Nephrology. 2012. №11, p. 6-15.
- Dvoretsky L.I., Karpova O.Yu., Aleksandrova E.N., Petrova S.Yu. Amyloidosis of the heart in the elderly. Archive of internal medicine. 2015. № 6 (26), p. 28-36.
- AmyloSENS: Removing junk from between cells ..
- 4Lee, V., Goedert, M., Trojanowski, J. (2001) Neurodegenerative tauopathies, Annu. Rev. Neurosci., 24, 1121–1159.
- Tatarnikova OG, Orlov MA, Babkova N.V. Beta-amyloid and Tau-protein: structure, interaction and prion-like properties. Successes of biological chemistry, t. 55, 2015, p. 351–390.
- Weller, R., Yow, H., Preston, S., Mazanti, I., Nicoll, J. (2002) Cerebrovascular disease is a major factor in the failure of elimination of amyloid beta from the aging human brain, Ann. NY Acad. Sci., 977, 162–168.
- Nalivaeva, N., Fisk, L., Belyaev. N., Turner, A. (2008) Amyloid-degrading enzymes as therapeutic targets in Alzheimer's disease, Curr. Alzheimer Res., 5, 212–224.
- Fisk, L., Nalivaeva, N., Boyle, J., Peers C., Turner A. (2007) Effects of hypoxia and oxidative stress on expression of neprilysin in human neuroblastoma cells and rat cortical neurons and astrocytes, Neurochem. Res., 32, 1741–1748.
- Jaunmuktane Z., Mead S., Ellis M., Wadsworth J. D. F., Nicoll A. J., Kenny J., et al.. (2015). Evidence for human transmission of amyloid-β pathology and cerebral amyloid angiopathy. Nature 525, 247–250.
- X-L Bu, Y Xiang, W-S Jin, J Wang, L-L Shen, Z-L Huang, K Zhang, Y-H Liu, F Zeng, J-H Liu, H-L Sun, Z-Q Zhuang, S-H Chen, X-Q Yao, B Giunta, Y-C Shan, J Tan, X-W Chen, Z-F Dong, H-D Zhou, X-F Zhou, W Song & Y-J Wang. Blood-derived amyloid-β protein induces Alzheimer's disease pathologies. Molecular Psychiatry. 2017.
- В.Ю. Лобзин, А.Ю. Емелин, Л.А. Алексеева. Ликворологические биомаркеры нейродегенерациив ранней диагностике когнитивных нарушений. Вестник Российской военно-медицинской академии. 2013, №4, с. 15-20.
- Blennow, Kaj, and Henrik Zetterberg. «Biomarkers for Alzheimer disease–current status and prospects for the future.» Journal of internal medicine (2018).
- Nabers, Andreas, et al. «Amyloid blood biomarker detects Alzheimer's disease.» EMBO molecular medicine (2018).
