Ask Ethan No. 36: An Amazing Rotating Electron
- Transfer
The reader asks:
There is a lot to be said about this, so let's start with the principle of Pauli’s prohibition.
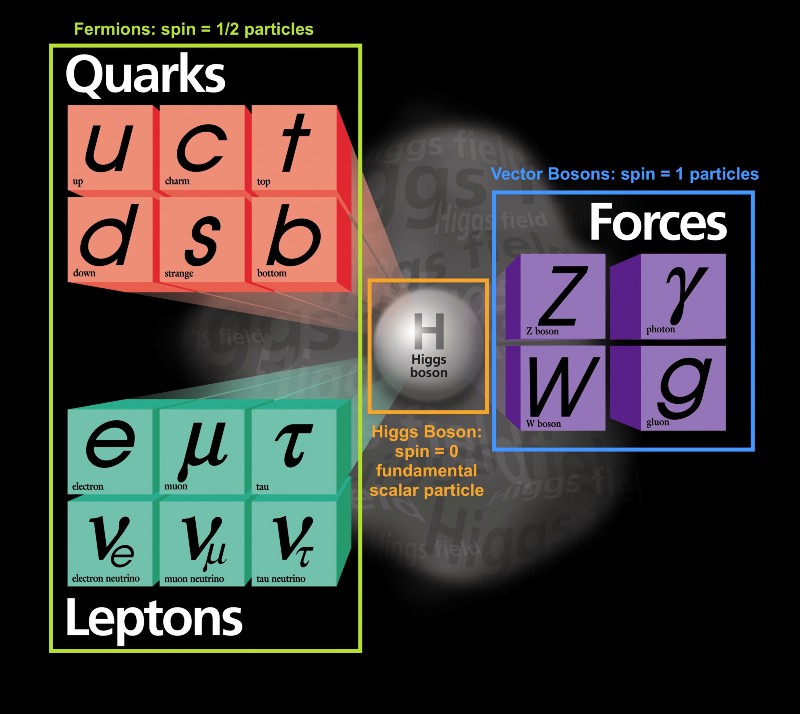
Despite the wide variety of different types of elementary particles that exist in the Universe, they can all be divided into two types:
Interestingly, composite particles also behave either as fermions or as bosons. Protons and neutrons behave like fermions with spins ± 1/2, like electrons. Each particle has a set of quantum states that it can occupy, with discrete energy levels, angular momenta, spin directions, etc.
The main difference between fermions and bosons is that if you have two identical particles, then you can send as many bosons in the same quantum state there, but identical fermions cannot occupy the same state.
If the electron were not a fermion, but a boson, then any atom could be crammed into any atom at any lower energy level (red above). But an electron is a fermion, so it obeys the principle of prohibition. Two electrons can take the minimum energy level, since they can have spins +1/2 and -1/2, but in order to add a third electron, you have to jump to another quantum state.
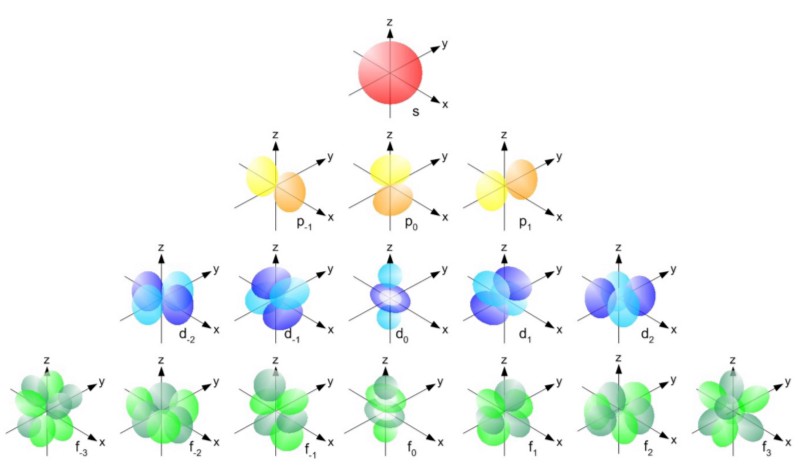
Quantum states in atoms are arranged so that you can go to a higher energy level (n in the picture below), and then to states with a higher angular momentum (l).
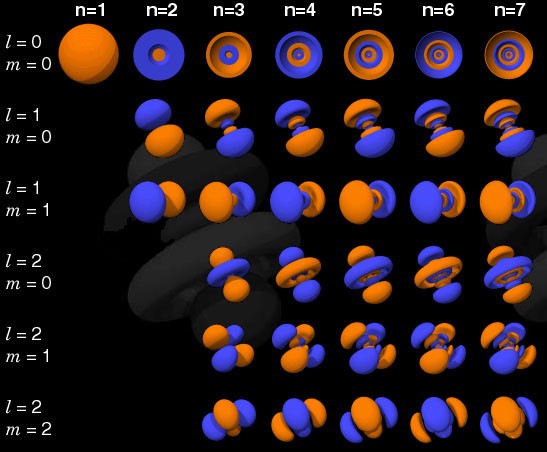
Therefore, the states l = 0 are s-orbitals, l = 1 are p-orbitals, l = 2 are d-orbitals, and so on. Therefore, the periodic table has just such a structure: with two elements in the upper row (n = 1, l = 0, m = 0 and spin = ± 1/2), 8 elements in the second row (n = 2, l = 0, m = 0, and spin = ± 1/2, and n = 2, l = 1, m = 1,0, or -1 and spin = ± 1/2), 18 elements in the third row, etc.
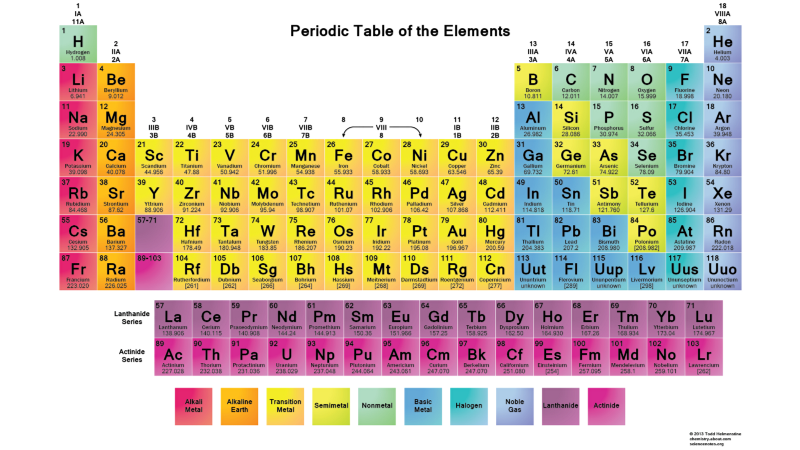
Therefore, adding an additional 6, 10, 14, etc. occurrences with each new row of the table is due to the Pauli principle.
And although we cannot distinguish one electron from another, since they are identical, each atomic system is unique. In other words, if you have four different hydrogen atoms in the ground state, they will not need to occupy different energy levels.

In general, since atomic nuclei (protons) differ from each other (are not in the same nucleus or are in overlapping quantum states in any sense), and electrons are attached to their proton (that is, they are not in overlapping quantum states with each other), a system of free hydrogen atoms is most likely organized in such a way that they will all be in a basic state, something like this:
At the very least, it’s wise to set up your system this way from the very beginning. But if a pair of such atoms interact with each other, they will unite and form a hydrogen molecule. Just like a hydrogen atom in its ground state is slightly lighter (13.6 eV) than a free proton and free electron due to binding energy, so a hydrogen molecule is slightly lighter (4.52 eV) than two free hydrogen atoms .
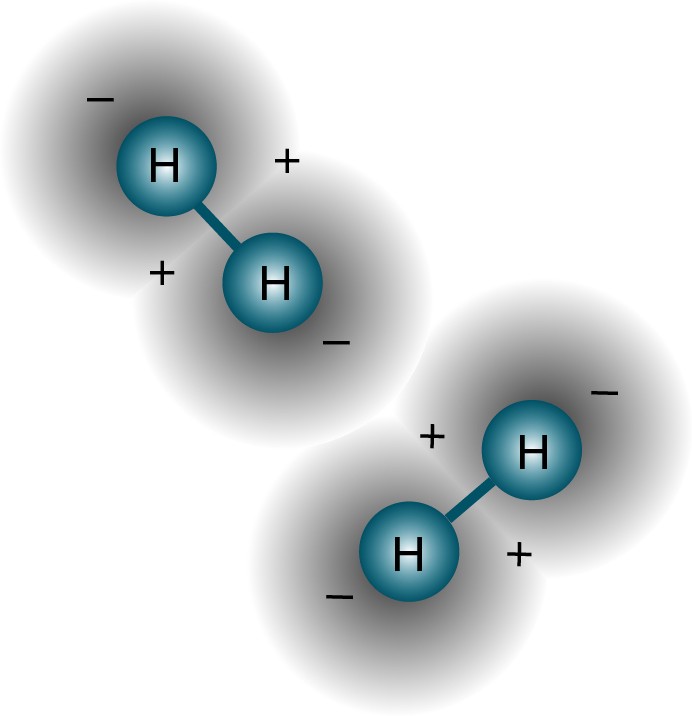
But the question was asked correctly. Since if two different atoms try to reconnect, the wave functions of the electrons will try to overlap each other.
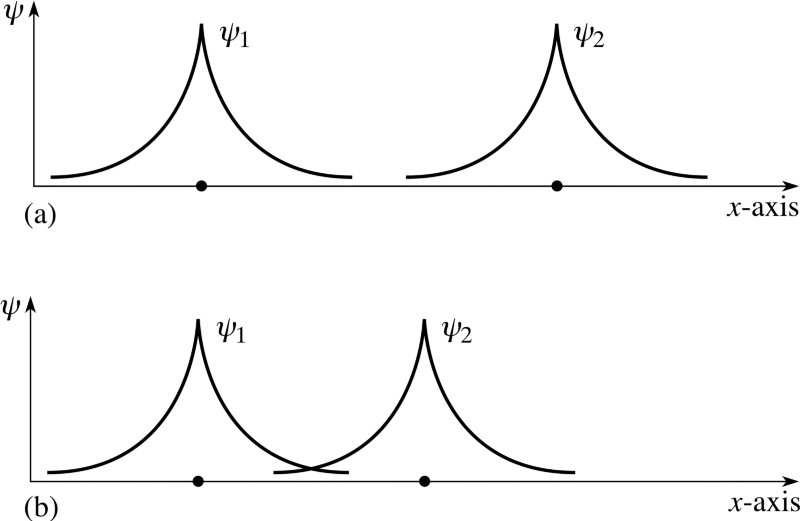
But electrons have not only spin, but also spatial wave functions. This means that they occupy space in a special way. If I bring two hydrogen atoms together, their spatial wave functions can be symmetric, as in the diagram above, or antisymmetric, as in the diagram below.
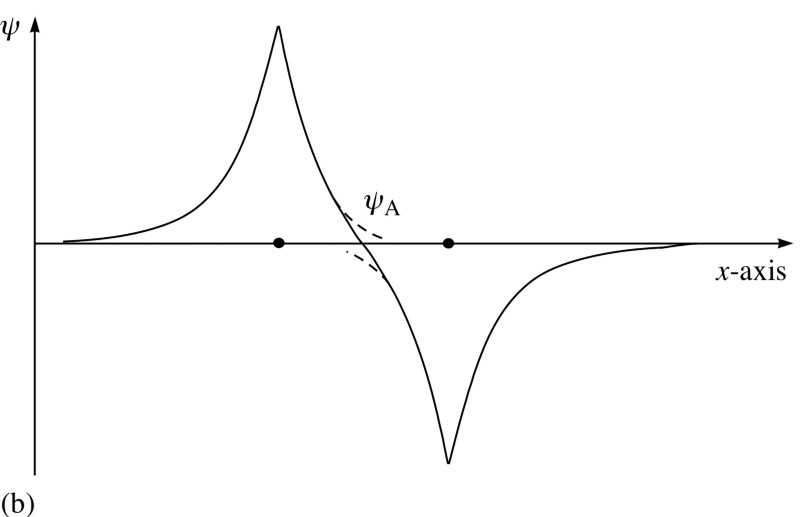
And here the Pauli principle comes into force.
If hydrogen atoms approach symmetric wave functions, then the spins of the electrons must be antidirectional - if one has a spin of +1/2, the second has a spin of -1/2, and vice versa.
And if two atoms come together with antisymmetric wave functions, then the spins of the electrons should be aligned: if the first one is +1/2, then the second one should also have +1/2, and vice versa.
Therefore, hydrogen atoms can be connected in two ways - either with symmetric wave functions and antidirectional spins, or vice versa.
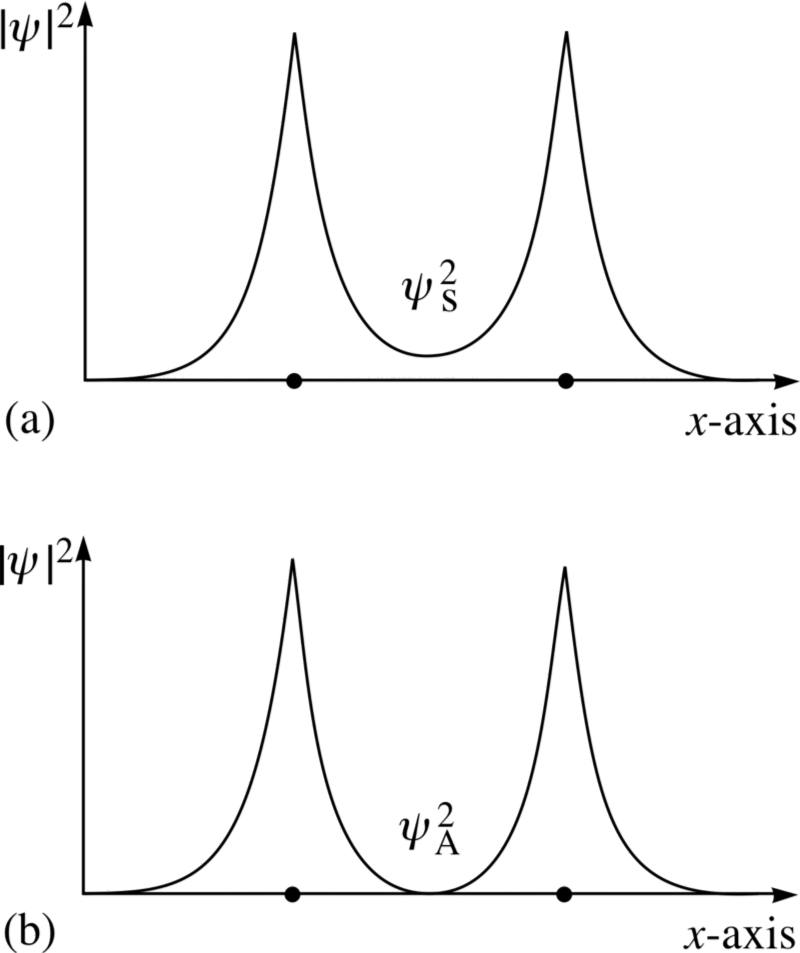
Take a look at these two combinations - at the top, the wave functions overlap, denoting a connection, and at the bottom, they do not overlap, which indicates that this state is not connected.
We can calculate what the binding energy for these two states will be.
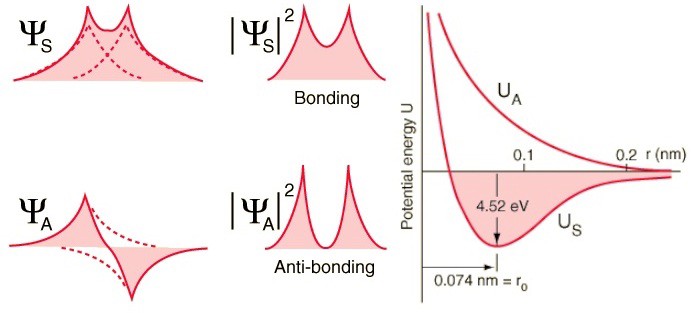
In an anti-symmetric state, where the spins of the electrons are aligned, atoms will not form. Only in a symmetric state, where the spatial wave functions are symmetrical and the spins are oppositely directed, can a hydrogen molecule form.
Therefore, to form a molecule, you need two hydrogen atoms with symmetric spatial wave functions and multidirectional spins (+1/2 and -1/2). And besides, you can see how quantum mechanics forbids you to push a third hydrogen atom into it - therefore, you can make an H atom, an H2 molecule, but never H3.
When I read your answer about lasers, I remembered my old question about the Pauli principle. As I understand it, for two electrons in a hydrogen molecule, the spins should be opposite. Does this mean that during the formation of a molecule, electrons change their spin, or can only electrons with opposite spins form a molecule?
There is a lot to be said about this, so let's start with the principle of Pauli’s prohibition.

Despite the wide variety of different types of elementary particles that exist in the Universe, they can all be divided into two types:
- fermions are particles with a half-integer spin: ± 1/2, ± 3/2, ± 5/2, ..
- bosons are particles with a whole spin: 0, ± 1, ± 2, ..
Interestingly, composite particles also behave either as fermions or as bosons. Protons and neutrons behave like fermions with spins ± 1/2, like electrons. Each particle has a set of quantum states that it can occupy, with discrete energy levels, angular momenta, spin directions, etc.
The main difference between fermions and bosons is that if you have two identical particles, then you can send as many bosons in the same quantum state there, but identical fermions cannot occupy the same state.

If the electron were not a fermion, but a boson, then any atom could be crammed into any atom at any lower energy level (red above). But an electron is a fermion, so it obeys the principle of prohibition. Two electrons can take the minimum energy level, since they can have spins +1/2 and -1/2, but in order to add a third electron, you have to jump to another quantum state.
Quantum states in atoms are arranged so that you can go to a higher energy level (n in the picture below), and then to states with a higher angular momentum (l).

Therefore, the states l = 0 are s-orbitals, l = 1 are p-orbitals, l = 2 are d-orbitals, and so on. Therefore, the periodic table has just such a structure: with two elements in the upper row (n = 1, l = 0, m = 0 and spin = ± 1/2), 8 elements in the second row (n = 2, l = 0, m = 0, and spin = ± 1/2, and n = 2, l = 1, m = 1,0, or -1 and spin = ± 1/2), 18 elements in the third row, etc.

Therefore, adding an additional 6, 10, 14, etc. occurrences with each new row of the table is due to the Pauli principle.
And although we cannot distinguish one electron from another, since they are identical, each atomic system is unique. In other words, if you have four different hydrogen atoms in the ground state, they will not need to occupy different energy levels.

In general, since atomic nuclei (protons) differ from each other (are not in the same nucleus or are in overlapping quantum states in any sense), and electrons are attached to their proton (that is, they are not in overlapping quantum states with each other), a system of free hydrogen atoms is most likely organized in such a way that they will all be in a basic state, something like this:

At the very least, it’s wise to set up your system this way from the very beginning. But if a pair of such atoms interact with each other, they will unite and form a hydrogen molecule. Just like a hydrogen atom in its ground state is slightly lighter (13.6 eV) than a free proton and free electron due to binding energy, so a hydrogen molecule is slightly lighter (4.52 eV) than two free hydrogen atoms .
But the question was asked correctly. Since if two different atoms try to reconnect, the wave functions of the electrons will try to overlap each other.

But electrons have not only spin, but also spatial wave functions. This means that they occupy space in a special way. If I bring two hydrogen atoms together, their spatial wave functions can be symmetric, as in the diagram above, or antisymmetric, as in the diagram below.

And here the Pauli principle comes into force.
If hydrogen atoms approach symmetric wave functions, then the spins of the electrons must be antidirectional - if one has a spin of +1/2, the second has a spin of -1/2, and vice versa.
And if two atoms come together with antisymmetric wave functions, then the spins of the electrons should be aligned: if the first one is +1/2, then the second one should also have +1/2, and vice versa.
Therefore, hydrogen atoms can be connected in two ways - either with symmetric wave functions and antidirectional spins, or vice versa.

Take a look at these two combinations - at the top, the wave functions overlap, denoting a connection, and at the bottom, they do not overlap, which indicates that this state is not connected.
We can calculate what the binding energy for these two states will be.

In an anti-symmetric state, where the spins of the electrons are aligned, atoms will not form. Only in a symmetric state, where the spatial wave functions are symmetrical and the spins are oppositely directed, can a hydrogen molecule form.
Therefore, to form a molecule, you need two hydrogen atoms with symmetric spatial wave functions and multidirectional spins (+1/2 and -1/2). And besides, you can see how quantum mechanics forbids you to push a third hydrogen atom into it - therefore, you can make an H atom, an H2 molecule, but never H3.

