Scientists have developed smartphone-controlled cells that deliver insulin to the blood

Smartphones can already control homes and cars, as well as diagnose diseases. A group of Chinese and Swiss researchers proposed to transfer to a smartphone the control of artificial cells implanted in the body to produce insulin. People with diabetes are forced to inject insulin on a daily basis (some weekly). A new device tested on mice may one day eliminate the painful need for needles.
Cell therapy is a new radical promising treatment option. The idea is to create by genetic engineering cells that are capable of secreting the necessary medicinal substances and implanting them into the body ... For example, white blood cells forced to fight cancer, HIV and other diseases. Hundreds of cell therapies undergo clinical trials, although not one of them has been controlled from outside the body.
Researchers have demonstrated a smart, closed-loop system in which a digital blood glucose meter transmits blood glucose data from mice to a smartphone. The smartphone processes the data and sends a signal to the implanted cells for insulin delivery. Less than two hours after cell activation, the blood sugar level in animals stabilized.
It would be impossible to create such an instrument without optogenetics, a research area that uses photosensitive proteins to regulate biological activity in the body. This method has been proposed for the treatment of a number of diseases, including Parkinson's disease and schizophrenia. The first clinical trial of achievements in the field of optogenetics , which is currently underway, is aimed at restoring the vision of a patient with retinitis pigmentosa - a degenerative state of the eye that leads to blindness.
As part of an experiment with diabetic mice in the first stage, the researchers changed human cells using a photosensitive gene, which was found in plants and causes the cells to produce insulin by signal. Scientists then injected photosensitive bacterial proteins into mammalian cells. Under the influence of far red light at a wavelength of about 730 nm, the protein activated a sequence of genes that caused cells to produce insulin.
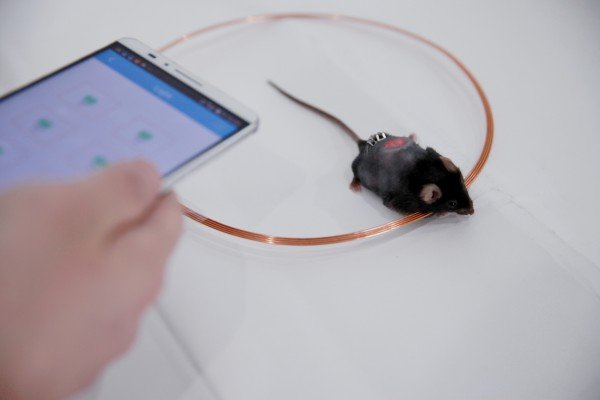
After a successful experiment, the researchers created a device the size of a ruble coin, in which receiving coils surround a hydrogel with built-in cells with red LEDs. These devices were implanted under the skin of diabetic mice. When the external coil wirelessly turns on the LEDs by electromagnetic induction, their light activates the cells that produce insulin.

The team of scientists did three things to remotely control the cells: a customizable Bluetooth-enabled blood glucose meter, an Android smartphone app and an intelligent control unit that controls the transmitting power of the coil.
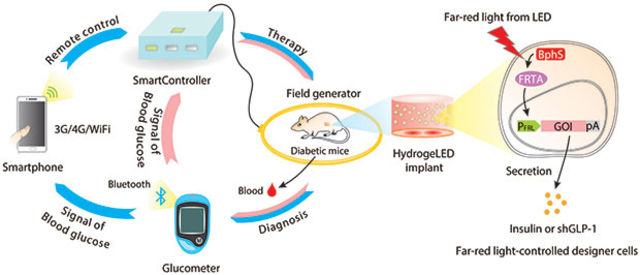
When researchers place blood samples of mice on a meter, it sends measurements to a smartphone via Bluetooth. The application compares these levels with a predetermined threshold, then transmits a signal to the control unit to turn on the power transmitter coil, which causes the diodes to glow long enough for the implant to deliver the right amount of insulin.
The application allows the user to determine how brightly the LEDs should glow, and how long they will control the amount of insulin in the cells. A Bluetooth transmitter connected to the meter can send a notification to the smartphone when the sugar level is too high and automatically turn on insulin production.
The blood glucose level of animals usually decreased to normal levels within two hours after the procedure. The system maintained a blood glucose concentration in mice for 15 days without any side effects. However, the researchers are sure that it is necessary to further study how much longer operation and the frequency of implant replacement affect the body and the operation of the device.
The system as a whole also requires significant refinement. The smartphone application actually “communicates” with the server, which is a kind of smart home center that includes an induction coil surrounding the mice with an electromagnetic field. Electromagnetism activates the LEDs in the implant, so it works only when the mice are near the transmitter, which can be a problem for any diabetic who wants to at least sometimes leave the house.
In addition, the current design still requires the use of a needle to check blood sugar levels. Future versions of HydrogeLED, researchers suggest, are designed to solve both problems. One of the authors of the study, Haifeng E, suggests the presence of a built-in glucometer, which 24 hours a day monitors the blood sugar level of the patient, automatically starting battery-powered LEDs when insulin is needed.
Scientists have a long way to go before HedrogeLED can be tested in humans. First you need to test on more animals - the current version of the technology was tested only on groups of five to six mice, as well as on larger animals, such as dogs or monkeys, for two to three weeks. Researchers must also ensure that all materials used are safe and do not stimulate immune responses.
doi: 10.1126 / scitranslmed.aal2298
