The logic of thinking. Part 14. Hippocampus

This series of articles describes a wave model of the brain that is seriously different from traditional models. I strongly recommend that those who have just joined start reading from the first part .
The complete removal of the hippocampus makes it impossible to form new memories, as the case with the HM patient convincingly demonstrated. Hippocampal abnormalities can lead to Korsakov’s syndrome, which also reduces the inability to record current events while preserving the old memory. All this convinces us that the hippocampus plays a key role in the memory mechanism.
Traditional theories about the role of the hippocampus are based on the analogy of the brain and computer. In such considerations, the hippocampus is given the role of "random access memory", that is, the place where new memories accumulate. Then, presumably in a dream, these memories are transferred to the areas of the brain responsible for the storage of long-term memory. Although the mechanisms of such a transfer are not clear, at least this helps explain why the disruption of the hippocampus blocks the formation of event memory.
Our model is fundamentally different from traditional models. We do not have any buffering of the memory in the hippocampus and its subsequent copying. Elements that form memories are immediately formed where they are appropriate. The hippocampus simply creates a single identifier that combines the memory elements distributed over the space of the cortex. The wave model of the brain explains how this identifier spreads throughout the cortex. The presence of such an identifier allows, by choosing the elements combined by it, to reproduce not an abstract picture, but a concrete image of a remembered event.
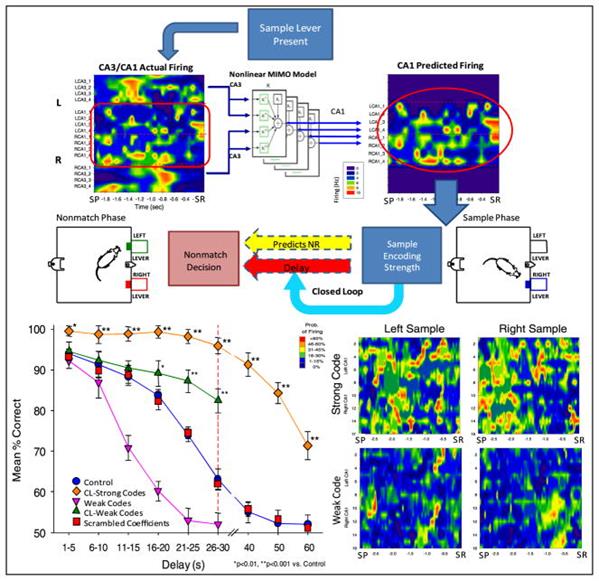
In 2010, interesting experiments were performed on prosthetics of the rat hippocampus (Theodore W. Berger, Robert E. Hampson, Dong Song, Anushka Goonawardena, Vasilis Z. Marmarelis, Sam A. Deadwyler, 2011). Microelectrode arrays encompassing the CA3 and CA1 regions were introduced into the hippocampus in experimental animals from both sides of the brain. After the rats recovered from the operation, a series of tests began.
Rats were placed in a chamber with two retractable levers. The test consisted of three phases. At first one of the levers was randomly advanced. When the rat pressed this lever, a picture of the activity of previously selected hippocampal neurons (sample stage) was recorded. Then the lever retracted, and there was a pause from 1 to 30 seconds in some series and up to 60 in others. Then both levers advanced. When the lever opposite the original sample was pressed, reinforcement was issued in the form of a droplet of water. If an error occurs, that is, pressing the same lever that was at the sample stage, the punishment was followed - turning off the light for 5 seconds. As a result, a plot of the success of attempts on the delay time was compiled (figure below).

Control measurements of the natural activity of the hippocampus (Theodore W. Berger, Robert E. Hampson, Dong Song, Anushka Goonawardena, Vasilis Z. Marmarelis, Sam A. Deadwyler, 2011)
Based on the recorded signals, a predictive model was constructed that predicted which signal should wait on CA1 depending on the signal on CA3. In a series of experiments, prediction signals were added to existing signals (Figure below).
Adding Artificial Signals (Theodore W. Berger, Robert E. Hampson, Dong Song, Anushka Goonawardena, Vasilis Z. Marmarelis, Sam A. Deadwyler, 2011)
An interesting result was obtained. It turned out that a significant influence on the result is exerted only when the signal is adjusted at the stage of presentation of the sample, i.e., when the initial image is stored. The picture of the signals at the remaining moments was not fundamental. Moreover, the stronger the correction signal, the better the final result.
The authors of the experiment, explaining the result, proceeded from the idea that the hippocampus is encoding current information, preparing it for writing to memory. That the patterns of activity observed in the hippocampus carry an informational description of the events. Accordingly, amplification of the “correct” signal helps to improve the results of memorization.
In our model, the explanation of the results is somewhat different. It is clear that the identifier of the hippocampus is important only at the time of formation of memory. After the memory is created, the hippocampus is not required to play it. Strengthening the current hippocampal identifier has a positive effect on the power of memorization, but this is not the result of creating a clearer information picture, but simply highlighting the current memory against the background of the rest of the memory. If a normally functioning brain emphasizes all memories in this way, the final result will be zero.
The most interesting series of experiments was related to the suppression of the intrinsic activity of the hippocampus. For this, rats were given regular injections of the MK801 hippocampus into the CA3 region. MK801 blocks normal synaptic transmission using glutamate. It penetrates the ion channels of NMDA receptors sensitive to glutamate and disrupts their work.
Blockade of CA3 site expectedly worsened the results. But the supply of artificial signals that restore the expected picture of activity, has significantly improved the number of correct answers (figure below).

Replacing the natural hippocampal signal with an artificial one (Theodore W. Berger, Robert E. Hampson, Dong Song, Anushka Goonawardena, Vasilis Z. Marmarelis, Sam A. Deadwyler, 2011)
I am not inclined to interpret this result as a miraculous restoration of the neural description of what is happening in the hippocampus with the subsequent recording of this description in memory. Moreover, in the experiment, matrices with only 32 electrodes were used, of which about half were involved. Most likely, a random identifier was created, which made the formation of memory possible.
But not everything is so simple with the hippocampus. There are several facts that are quite perplexing when you first meet them. So in 1971, John O'Keefe discovered cells in the hippocampus (O'Keefe J., Dostrovsky J., 1971). These cells react like an internal navigator. If a rat is placed in a long corridor, then by the activity of certain cells it will be possible to say exactly where it is located. Moreover, the reaction of these cells will not depend on how it got to this place.
In 2005, neurons encoding a position in space were found in the hippocampus, forming something like a coordinate grid (Hafting T., Fyhn M., Molden S., Moser MB, Moser EI, 2005).
In 2011, it turned out that there are cells in the hippocampus that encode time intervals in a certain way. Their activity forms rhythmic patterns, even if nothing else happens around (Christopher J. MacDonald, Kyle Q. Lepage, Uri T. Eden, Howard Eichenbaum, 2011).
From all these facts, the conclusion suggests itself that the hippocampus is responsible for coding our position, both in time and in space. At the same time, the hippocampus is similar to a soldier, for whom it is quite natural to “dig from the fence to lunch”. The hippocampus combines both spatial and chronological navigation, which, incidentally, have much in common. Just as our journey in space proceeds from landmark to landmark, so a journey in time passes from one chronological mark to another.
We previously stated that the hippocampus forms unique identifiers for memories. How does this function relate to the fact that the hippocampus tracks our spatiotemporal coordinates? Of course, we can assume that the hippocampus has a dual function, participating in two independent processes. But it is more logical to assume that we are dealing with two sides of the same coin.
How do we identify information in general? Using Google, we create a query consisting of a set of words that defines the meaning of our search. Having received the list of results, we can then refine it by introducing restrictions on the date or geography.
Creating a library of images, we mark the photos with the date of their creation and geotagged places where they are made. Then we describe who or what is captured on them.
When placing files on a computer, we indicate the time of creation and the path to the file. By the name of the file we briefly describe its contents.
It turns out that identifying the most different things, we use, on the one hand, the signs that describe their meaning, and, on the other hand, the coordinates in space and time, as universal signs of any phenomena. Such identification subsequently turns out to be quite convenient and useful not only as a unique tag, but also as a search tool. It can be assumed that nature went the same way.
That is, creating a unique identifier for the event, the hippocampus makes sense not only to generate a random code and distribute it throughout the cortex, but to lay in this code spatio-temporal features, as the most suitable for all occasions. Such a spatio-temporal description in itself is quite unique, as a combination of various factors. It is enough to add a small random appendage to it and the resulting identifier will definitely indicate a specific event.
Such identification is much more powerful in its capabilities than just a unique key for each memory. The possibility of a variety of additional associations. But most importantly, it becomes possible to encode complex descriptions that take into account how events develop both in time and in space. But talk about this will be a little later.
If we assume that the identifier of the hippocampus really encodes the space-time coordinates, then it turns out that the hippocampus, firstly, has the ability to interact with the cortex in such a way as to be able to learn to recognize similar positions in space and time. Secondly, in order to spread its identifiers throughout the space of the cortex, the hippocampus must form them from a finite number of unique fragments. This stems from the fact that the cortex must be preliminarily trained to distribute the entire “alphabet” used by the hippocampus. New identifiers should be formed not “from scratch”, but as combinations of already known elements. In principle, there is nothing complicated in these requirements, this corresponds to how in phrases in a natural language all phrases are built from a finite set of letters.
Used literature
Continuation
Previous parts:
Part 1. Neuron
Part 2. Factors
Part 3. Perceptron, convolution networks
Part 4. Background activity
Part 5. Brain waves
Part 6. Projection system
Part 7. Human-computer interface
Part 8. Isolation of factors in wave Networks
Part 9. Patterns of neuron detectors. Rear projection
Part 10. Spatial self-organization
Part 11. Dynamic neural networks. Associativity
Part 12. Traces of memory
Part 13. Associative memory
Alexei Redozubov (2014)
