Use and restoration of lead batteries my experience

Recently, I did another battery replacement in my UPS. I decided to study more deeply the question of the correct use of lead batteries, their design and the chemistry of the process.
The price of batteries is increasing due to the course and it becomes expensive to buy them.
Is it possible to make batteries last longer? How do I get the most out of them so that the equipment lasts longer and the power outages do not bother me at all?
I want to share my experience. Who cares, I ask under the cat ...
Long battery life.
On the batteries for the UPS, manufacturers write a 20-hour capacity, that is, the capacity that the battery will give for 20 hours of discharge.
But in bespereboynik such mode does not happen. They work on battery for about 30 minutes at best. And usually 5-10.
Let's look at the datasheet label on the CSB GP1272 battery with the stated capacity of 7.2 Ah:
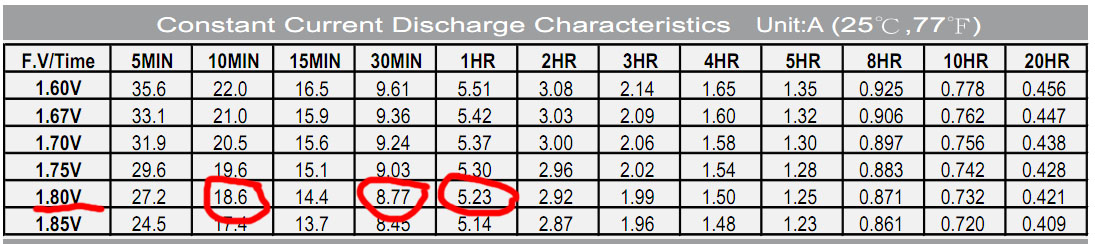
So, if we discharge it 1 hour to 10.8 volts (it is no longer recommended to lose the resource anymore), then it will give 5.23 Ah. Already very far from the stated 7.2 is not it?
If 30 minutes, then 4.38
If 10 minutes, then 3.1 is only 43% of the capacity!
Conclusion: lead batteries do not like to give large currents.
Let us leave the declared capacity on the manufacturers conscience and think how best to proceed.
That's how:
Bespereboyniki with one battery to power the computer is not suitable. Well, maybe except for very weak office machines.
Well, or they will work in minutes, and the batteries will die quickly and not give up a third of their capacity.
The uninterruptible power supply unit itself can withstand for some time the 300W load that is written on it, but the battery inside will be very hard.
Such bespereboynik are suitable for powering some low-power device (router for example) or nettop or very weak office sistemnik with a small monitor.
To power the computer, you need to use a battery backup unit with two batteries. Usually these are “smart” category devices.
This is not only 2 times the capacity, but 2 times less current. So, give the batteries will be able to amp * hours more.
It is also a good idea to use high-quality power supplies with a PFC corrector and high efficiency in a computer.
With a good power supply will be less loss, which means longer battery life.
Long life battery
Why do some batteries last for 5-6 years in some bespereboynik, while others die for a year, and they have to be picked out by the installer? Let's try to figure it out.
To do this, let's look at this graph from the datasheet:

Now let's take a thermometer and measure the temperature in the room and in the battery compartment.
Let's look at the chart now. If the temperature of the batteries is 20-25 degrees (as usual indoors), then the service life is 5 years. If 35, then 2 times less! And if above 40, then the battery will live less than 2 years.
Conclusion: the batteries must be cold! Well, that is not above room temperature.
With increasing temperature, chemical processes and electrolyte evaporation are accelerated.
And yet, there is still such a thing as temperature compensation of the charge voltage.
Some datasheets indicate it. But more often simply lead modes for 20 or 25 degrees Celsius.
Here is a datasheet diagram:
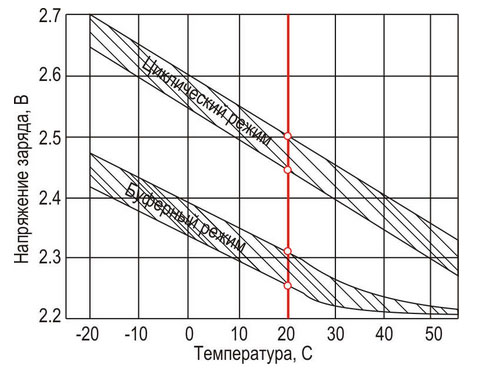
The charge voltage for different temperatures is different and must be adjusted according to the actual temperature in the battery compartment. Advanced UPS can do it themselves. But more often, the charger is stupid there and boils the batteries with an increased charge voltage in addition to heating them.
Let's see how things are in real devices
I have 2 smart-type UPSs. One Ippon is like this:

And another APC smart 700 is like this:

Well, a couple more unpretentious APC back CS500.
Smart devices have one feature. There stands the Big Iron Transformer (BAT).
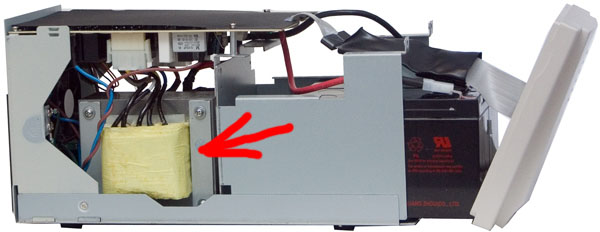
It is active whenever the UPS is plugged in. And it is heated! When powered from the network, this BAT operates in the autotransformer mode and can increase or decrease the voltage by switching the windings. Just as in transformers for the lamp grandfather TV, but only automatically. From him is charging. Although in more modern UPSs, charging is done on a separate impulse.
So in Ippon this transformer transfers 30 watts of heat into the heat. And in the APC is almost 20.
(Measured consumption at idle)
I measured the temperature in the room, as well as the temperature in the battery compartment of the UPS.
I also measured the charge voltage.
It turned out this:
The temperature in the room is 25 degrees.
The temperature in the UPS Ippon 25 degrees.
The temperature inside the APC is 34 degrees!
The charge voltage is Ippon 27.5 V, the APC has 27.2 V.
The Ippon has a cooler. And it turns whenever it is turned on. Designers took care of cooling, despite the fact that this is not the coolest manufacturer. But the charger there is the simplest linear on the LM317. And the voltage is high for my 25 degrees indoors.
APC is in a bad situation. Forced cooling is absent, the installation is tight, the transformer heats the battery compartment. And although the charge voltage is roughly equivalent (perhaps there is even a temperature correction), it will still quickly kill the batteries.
What will i do
In Ippon, I will slightly reduce the charging voltage. Make it easy. It is enough to calculate and solder the resistor in the LM317 divider chain. So I did. Now the voltage is 27.15V.
In the case of APC, I decided to install a cooler there. You can of course remove the battery from the case. But such a decision did not seem to me aesthetic. Besides, components of the UPS will be better cooled, condensers will not dry.
Take the plumbing tool and go:


Well, in small APC back CS500, you don’t need to do anything. There is a pulse charger, and it almost does not heat up. Voltage within normal limits.
So, for a long battery life you need
Provide temperature control.
In the case of a server room, it is the removal of batteries into a separate room, a cabinet, a box and the provision of ventilation / cooling.
In the case of a regular uninterrupted power supply, this is the introduction of a cooler, the removal of batteries beyond the hot case.
Ensure that the charging voltage and temperature of the batteries.
Adjust charge voltage if necessary.
Battery Test and Recovery
Now it became interesting to me. Is it possible to try to restore the used batteries? Dried and lost capacity.
It is clear that on the Internet a lot of nonsense and fake. I decided to start a little to explore the essence of the issue and read the theory.
I read the book that's it . And here . And still article on Habré .
Conclusions from reading
- There can be no miracle. You can try to recover only relatively lively batteries with certain symptoms. If the battery banks are short-circuited, the plates fall off or fall off, then there is nothing to do. Only in color!
- The recovery process is very long (about a week for one battery). Therefore, it is very labor-intensive to do this “by hand” and does not make sense even at the stage of experiments. Only an automated process makes sense.
- You can try to restore the batteries that worked in bespereboynik. Because the main reasons for the loss of capacity of these batteries are the loss of water as a result of constant recharging and sulphation due to non-optimal charge and discharge modes.
- Charge and discharge the battery is better pulses. So less boils and crystals of the correct structure are formed.
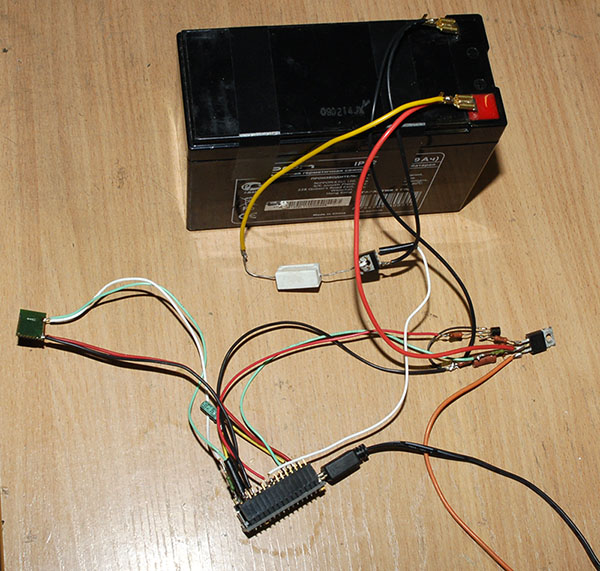
I made an experienced installation option to test and restore the battery.
Here is its scheme: Clickable As a “brain” I took the Arduino nano. Current source - laboratory power supply with control of current and voltage. For communication with the outside world - Bluetooth module HC-05. Key Q1 connects charging. Q3 connects the load R4 for discharge. R6 / R8 divider to control the voltage on the Arduino ADC. The main idea of this installation is that it works somewhere in the far corner by itself, does not ask / eat / drink. Sometimes you can look at what is happening there and even do not need to approach it. So far, everything is done "on snot." I do not know whether it will make sense of all this, so I didn’t bother with the board and the case yet. All this trouble is controlled remotely from the terminal:
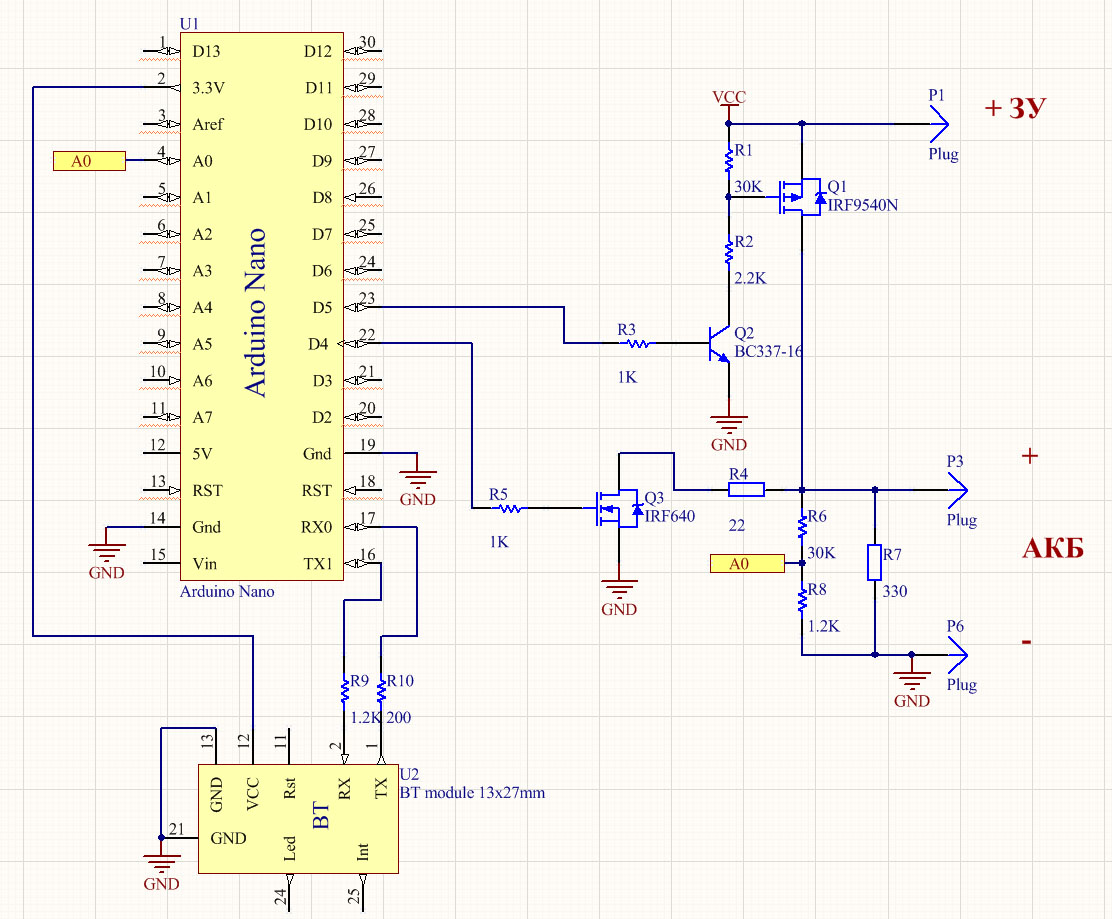
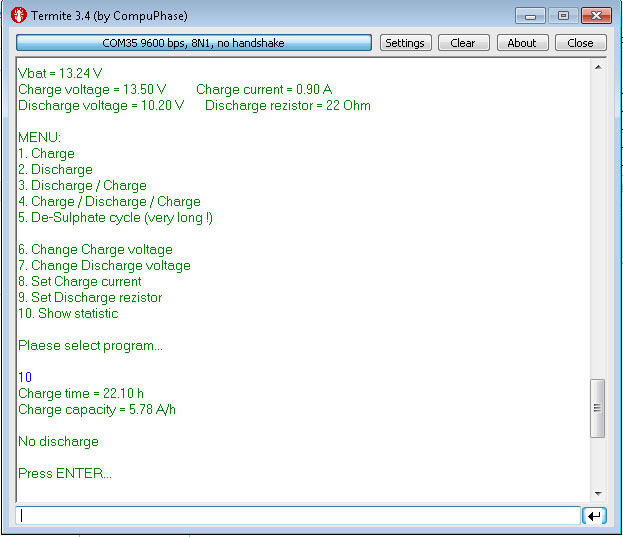
The scheme allows you to run different charge / discharge cycles according to the program and considers approximately how much electricity was spent on the process. You can determine how much the battery takes and gives.
The operation algorithm is as follows:
The charge goes by impulses 0.5 seconds charge and 1 second relaxation.
Discharge impulses 1/1 sec.
Voltage measurement during charging is in pauses (not energized)
Voltage measurement during discharge occurs under load.
We charge or discharge for 3 minutes, then we measure the voltage, send the data to the Bluetooth module and decide whether to continue.
There is also a desulfation program. It is long.
First 3 cycles of "alignment". This is a small current charge and waiting 10 hours.
Then discharge / charge cycles.
Choose "guinea pigs"
Battery No. 1 Sven. (Her photo at the beginning of the article)
This is a 2012 battery. The UPS does not swear at it, passes the self test, but it has almost no capacity left. She keeps 10 minutes bespereboynik, loaded on the router. She was new and rubbish, and after 6 years of work there were horns and legs :) But for bullying - the most it.
Opening the lid and peering into the jars revealed that the battery is badly short of electrolyte.
Battery No. 2 Ippon

She 2014, worked in a smart-type UPS up until the next battery in a pair had a short circuit in the bank. It happened recently. That is, the operating time of more than 4 years. She pretty much boiled and had to pour water into it.
Top up with distilled water
It is water, not electrolyte. Because it is water that leaves, and sulfuric acid remains on the plates in a bound state. Plain tap water will kill the battery immediately.
It is necessary to
add this way: We add charged battery. Because during the work, the electrolyte level changes and in the charged state it is maximum. To avoid overflow.
Use a syringe with a blunt needle to drip water directly onto the plates. And look at the flashlight.
It is necessary that the plates were wet from above, but that the water does not flop.
The procedure is repeated 2-3 times as water is absorbed after a few hours.
I poured about 50 ml of water into the tested battery No. 1. Very much, the battery was almost dry! In battery number 2 I refilled a little less, but also 6-8 cubes in each jar.
After topping up the water, the voltage dropped. Water involved parts of the plates, which were dry for a long time and it is not clear on what deposits.
So, let us suppose that a battery does not allow poorly soluble deposits to live normally (lead sulfate and α oxide of lead). They have a high resistance and passivate the plate sections. In addition, these deposits are dense and the electrolyte does not penetrate into them. The specific surface is small and electrolyte circulation is none. As a result, symptoms: loss of capacity of the battery, high internal resistance (the battery can not give a large current), boiling when charging.
A battery in this state can even give away its passport capacity. But only sooo small currents. So there is no practical benefit from this.
The task of the recovery cycle is to dissolve the “harmful” salts. And by charging with the correct mode, create new structures with the right structure.
Battery 1 required a long alignment. That is, charge cycles with waiting.
We charge, we wait, the voltage drops. Then we charge again.
I think that due to the prolonged boiling of water, uneven deposits with different properties formed on the plates. It turns out a different charge within the same plate.
Unfortunately, further tests of this battery for discharge / charge revealed that it has rotten plates in one of the cans. It is seen as a "step" on the discharge curve.
It looks like this:
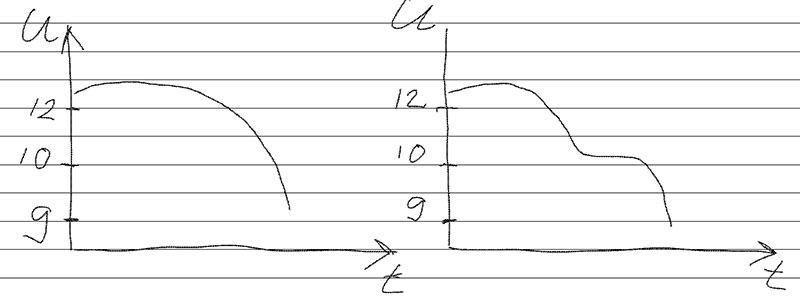
On the left - the normal bit curve. On the right - what happens when a part of the plates is peeled off.
Battery number 2 almost did not require alignment.
The electrolyte boiled quickly in it due to the accident of a nearby battery, and I assume that the hardly soluble deposits did not have time to form.
I drove her 2 cycles of discharge / charge.
For the test in conditions close to real, I used the APC Back CS500, loaded on a 60W light bulb. The power of the light bulb is known and measured, the efficiency of the UPS is also measured and equal to 80%. From the time of work it will be possible to calculate the output capacity.
Here is the test setup:

After topping up the water, but before the recovery cycles, I charged the No. 1 battery in a regular manner from UPS and discharged it to the light bulb.
The lamp was on for 8 minutes, and the battery was discharged to 9.5 volts (measured under load). Then uninterruptible disconnected. Take these 8 minutes as a starting point (before recovery procedures).
I did not torment the battery number 2 until recovery. It is still fit, and it can be killed by discharge to 9.5.
After the restoration, I tested the battery number 1 on the same stand with a light bulb and ...
it lasted 16 minutes.
That is 2 times longer than before. And this is with an average current of 6.5A.
Of course, the rotten plates could not be saved, but I liked the dynamics.
Even this dead battery can be used to power any router or switch somewhere in the attic / basement and it will hold for 30-40 minutes.
Given capacity up to 0.87 Ah, after 1.73 Ah
Battery # 2, after recovery, stayed on the stand with a light bulb for 37 minutes.
In this case, I discharged it not to 9.5 and to 10.5 volts. It is calcium and cannot be discharged to 9.5.
The given capacity is 4 Ah at an average current of 6.5 A.
Let's compare this with a datasheet from above. Datashit of course to another battery, but it is not very important.
The table does not have a value of 6.5A, but there are adjacent columns for a voltage of 1.75v per element.
I approximately counted and it turned out 50 minutes would hold a new battery with a current of 6.5A on datasheet.
This means that the battery number 2 gives about 74% of the capacity of a relatively new. I consider it quite good after more than 4 years of work and an experienced accident.
This battery will still serve.
In general, I certainly do not recommend using remanufactured batteries for important tasks.
But for secondary, for powering low-power and non-critical equipment, they can be used.
I also plan to use the setup to run a test discharge / charge of used batteries about once a year. I will evaluate their capacity and suitability so as not to get an accident with the destruction, short circuit of the battery and burnout of the backup bus.
Thank you all for your attention, I hope someone will come in handy.
If anyone wants to donate a battery for experiments in Barnaul, please in PM.
